Publications
Teratology Primer, 3rd Edition
How Do Genes Affect the Risk of Having a Child with a Birth Defect?
John M. Graham, Jr.
Cedars Sinai Medical Center Los Angeles, California
Every infant has at least a 3%–5% risk of being born with either a malformation or deformation, and an even higher risk (approximately 10%) of being born with internal anomalies or functional deficits that may not become apparent until later in life. The cause of most congenital anomalies is unknown. Approximately 50% of malformations fall into the group of those defects with unknown cause(s). It has been estimated previously that genetic causes (anomalies arising from alterations in genetic material) account for at least 15-25% of all human malformations. As we have learned more about genetic factors, and developed new techniques, the proportion of birth defects attributed to genetic causes has increased.
There are numerous types of genetic alterations, the most common of which are mutations (changes to the DNA sequence of genes) and chromosomal defects (e.g., extra or missing chromosomes or parts of chromosomes, Figure 1). Genetic alterations leading to malformations can be inherited, or can occur spontaneously due to random mutations of DNA. To date, genetically mediated malformations have not been found to result from exposure to any environmental agents, even to those agents that have been shown to be capable of causing damage to genetic material in individual cells (mutagens). Radiation is a potent mutagen, but even after the atom bombs were exploded over Japan, a careful study of the exposed population over subsequent generations demonstrated no increase in birth defects or genetic diseases caused by new mutations, despite the significant radiation exposure. Individuals exposed to some highly mutagenic chemotherapy drugs also do not produce offspring with more than the expected incidence of birth defects and other genetic diseases. Environmental causes of human malformations (defined as any external influence on fetal development, i.e., not genetic) are thought to be rare. It is estimated that only 1% of all human malformations are related to drug exposures, chemicals, or radiation.
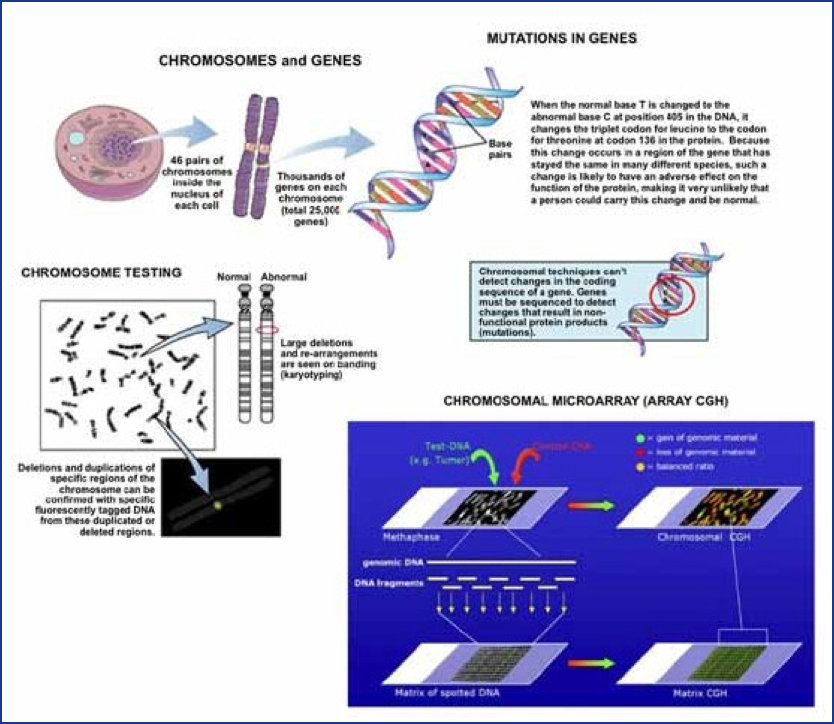
A birth defect can occur singly as an isolated defect. Multiple birth defects can also occur in one individual. When multiple birth defects, especially defects affecting different organs and systems appear together and are seen in different individuals in different families in a recurrent pattern or combination, they are generally accepted to have a common underlying cause and are designated as a birth defect syndrome. One example is Down syndrome, or trisomy 21, caused by the presence of all or part of an extra chromosome 21. The risk of having a child with Down syndrome is increased with increasing maternal age. Paternal age may also be a factor when the mother is 35 or older. Even with well-defined and refined syndromes, there is inherent variability in the manifestations of these birth defect syndromes, both in the type and severity of the various structural abnormalities that may appear. As a particular pattern of defects or syndrome is seen in additional patients and as more is learned about a syndrome over time, initial descriptions are refined. Patients with the same syndrome will manifest varying degrees of common core features as well as occasional unusual or infrequent (but related) features. The purpose of syndrome identification and refinement is to enable clinicians to recognize these features as suggesting a specific condition, with a common underlying cause, natural history, and prognosis. Syndrome diagnosis assists clinicians in, among other things, patient counseling and treatment, and this process has been facilitated in recent years through the use of single-nucleotide polymorphism (SNP) chromosomal microarrays (CMA) and whole exome sequencing (WES), which have now become frontline genetic tests. When compared with the standard genetic testing used previously, whole exome sequencing identified more conclusive diagnoses (29.3% to 52% of WES cases versus 7.3%) while CMA revealed diagnoses in 17% of cases. Such testing is now used routinely in clinical practice.
There are two broad classes of birth defect syndromes, genetic and non-genetic. Genetic syndromes result from some change in the genetic material of the conceptus, occurring prior to or around the time of conception. Suggested reading at the end of this chapter describes the general approach for determining the difference between these two broad classes of birth defect syndromes. An alteration in the genetic material of the cells may cause a genetic disease or genetic birth defect syndrome. While the same genes and the same genetic alteration will generally appear in each cell of the body, not all genes are expressed (i.e., activated) within each tissue in the body. The expression (or lack of expression) of each gene or group of genes in a given tissue is often controlled by epigenetic mechanisms and is responsible for different functions in different cells. When an error occurs in genetic material affecting a specific gene(s), even though the error appears in each cell of the body, it will only affect those cells (and thus organs) in which those genes are expressed. The functional capabilities of other genes, not affected by the error, help to determine much of the individual variability among different people with the same syndrome.
Thus, syndromes occur within the context of the underlying genetic background for each individual. Among children with Down syndrome, parental background for common genetically determined traits like stature, intelligence, and pigmentation always come through in the child with the syndrome, so that the tallest children with Down syndrome come from the tallest parents. There are underlying genetically determined susceptibilities for many common birth defects, which help to explain why different individuals with the same syndrome do not have the exact same combination of birth defects.
The type and location of each specific genetic alteration within a given gene will determine the variability and severity within a specific syndrome. Likewise, it is believed that all humans carry some genetic alterations that do not cause problems, either because the function of that particular gene product is covered by the other member of the gene pair, because that particular mutation does not alter the function of the gene product that it encodes, or because the particular mutation occurs in a non-essential part of the genetic material.
Genetic alterations can occur in either the sperm or the egg, or both, prior to or at the time of conception. If there is an alteration in the genetic material of the sperm and/or the egg, when the sperm fertilizes the egg at conception, each cell that derives from that fused egg/sperm cell (zygote) will carry that mistake. It is also possible that the genetic material of the sperm and egg could be “normal,” but shortly (within hours, or at most days) after conception, a mistake occurs during cell replication. This type of cell replication error would result in “mosaicism,” meaning that only some cells of the body will carry the error or mutation (i.e., those cells that derive from the cell where the error first took place). Even when such a mosaic genetic error occurs after conception, the error must occur shortly after conception, since after the first week there would be far too many cells without the mutation for manifestations of the genetic alteration to be apparent. Some genetic mutations may not be compatible with survival of the early embryo and result in an early spontaneous abortion. A mutation that occurs within the male or female germ cells could result in the occurrence of a dominantly inherited syndrome, even though neither parent shows signs of the same syndrome. Spontaneous mutations are not uncommon. When neither parent has the dominant genetic problem seen in their child, it is termed a de novo (new) or sporadic occurrence. It is estimated that between 3.0 and 7.5% of all malformations in humans are the result of such fresh dominant mutations in the genetic material.
Genetic disorders can also be inherited from the affected individual’s normal parent(s). Within the 20,000 pairs of genes in the human genome, one member of each pair, or one allele, is derived from each parent. Because genes work in pairs, a person can have a functional gene or allele and a defective gene, and still be “normal.” Usually the functional version of the gene pair, where one gene is defective, allows that gene to perform its specific task. The gene that does not function in these cases is called a recessive gene. Carriers for Tay-Sachs disease, cystic fibrosis, or sickle cell disease can be without the condition but carry the defective gene because it is only in one copy. In normal carrier parents these recessive genes only become known when each parent has a similar mutation in the same recessive gene, and the child inherits both non-working genes (one mutant gene from each parent). The risk for two carrier parents to have a child with a recessive disorder is 25%.
Dominant conditions result when there is one defective gene in a gene pair, and the normal member of that gene pair cannot complete a specific developmental task by itself. These dominant conditions usually result in a pattern of birth defects, a specific birth defect, or a risk for specific types of cancer because these genes affect developmental pathways or basic cell replication pathways. When a person has a mutation in one of these genes, the chance of passing it to an offspring is 50%; thus, such dominant autosomal conditions are inherited in families. When such conditions appear for the first time in a family, they reflect a sporadic new occurrence of the condition.
To the extent of our current scientific knowledge, mutations leading to birth defects occur in the normal course of cell division. There is one exception: advancing age. Advancing maternal age is associated with an increased risk of nondisjunction resulting in chromosomal aneuploidy. Advancing paternal age is associated with an increased risk for structural chromosomal defects and gene mutations in the male germ cell. It is commonly accepted that fresh dominant mutations occur more commonly in the sperm than in the egg. The susceptibility of sperm stem cells to genetic damage may be an important factor in the accumulation of genetic damage since men produce spermatozoa continuously throughout their reproductive lives. In contrast, oocyte development arrests before birth. The male germ cell begins to show effects of aging at 30 years, and most in vitro fertilization centers will discourage a man older than 40 years from donating his sperm.
Clearly genes, and genetic alterations, play a role in the risk of having a child with a birth defect. Tests are available to screen prenatally for some of these genetic alterations. Furthermore, while the genetic make-up of the offspring is critical, the environment also plays a role in determining cell fates. The extent to which environmental exposures and interactions between genes and the environment affect the risk of birth defects are active areas of research new occurrence of the condition. In many instances, random or spontaneous mutations or chromosomal errors can lead to genetic birth defects and genetic birth defect syndromes.
Suggested Reading
American College of Medical Genetics, Evaluation of the newborn with single or multiple congenital anomalies; a clinical guide. www.acmg.net 1999.
Berg AT, Coryell J, Saneto RP, et al. Early-life epilepsies and the emerging role of genetic testing [published online JAMA Pediatr. 2017;171(9):863-871.
Brent RL. Environmental causes of human malformations: the pediatrician’s role in dealing with these complex clinical problems caused by a multiplicity of environmental and Genetic factors. Pediatrics 2004: 113:957–968.
Chervenak FA, McCollough LB, Brent RL. The peririls of the imperfect expectation of the perfect baby. Am J Obstet Gynecol 2010;203:101.e1-5.
Crow JF. Age and sex effects on human mutation rates: an old problem with new complexities. J Radiat Res. 2006: 47(Suppl): B75–B82.
Graham JM. Jr, and Sanchez-Lara PA. Smith’s Recognizable Patterns of Human Deformation, 4rd Edition, Philadelphia: Elsevier-W.B. Saunders Co., 2016.
Jones KL. and Jones MC. Chapter 43: A clinical approach to the dysmorphic child. In: Rimoin D.L., Connor J.M., Pyeritz R.E., Korf B.R. (Eds.), Emery and Rimoin’s Principles and Practice of Medical Genetics (7th Edition), New York:, Churchill Livingstone, pp 889–899, 2018.
Lemke JR. High-throughput sequencing as first-tier diagnostics in congenital and early-onset disorders. JAMA Pediatr. 2017;171(9):833-835.
Schardein JL., Chemically Induced Birth Defects (3rd Ed.), Marcel Dekker Inc, New York, 2000.
Tan TY, Dillon OJ, Stark Z, et al. Diagnostic impact and cost-effectiveness of whole-exome sequencing for ambulant children with suspected monogenic conditions. JAMA Pediatr. 2017;171(9):855-862.
Vissers LELM1, van Nimwegen KJM2, Schieving JH3, et al. A clinical utility study of exome sequencing versus conventional genetic testing in pediatric neurology. Genet Med. 2017;19(9):1055-1063.
