Publications
Teratology Primer, 3rd Edition
What Tests Are Available to Screen Prenatally for Birth Defects?
Sarah G. Običan University of South Florida, Tampa FL
Karla Leavitt University of South Florida, Tampa, FL
Anthony R. Scialli Reproductive Toxicology Center, Washington DC
There are two kinds of tests used in clinical medicine, screening tests and diagnostic tests. Screening tests identify people in the general population who have a higher than average risk of a disease of interest. Diagnostic tests address the question of whether a particular individual is affected. Often in obstetrics, a screening test is applied to all pregnant women to find those women at particular risk of having a baby with a congenital abnormality. Diagnostic tests are then used to see which of the women in fact is carrying an affected child. Most diagnostic tests in pregnancy are invasive, have some risk for pregnancy complications and fetal loss, and may not be desired by every patient or family. Screening tests, therefore, refine the population for which diagnostic tests may be worth the risk.
The ability of screening tests to correctly predict abnormalities varies depending on the incidence of the condition in the general population and the reliability (its ability to accurately deliver a result) of the test. Ideally, the test should have few false positive results (predicting that a normal pregnancy is abnormal) and few false negatives (predicting that an abnormal pregnancy is normal).
The background risk for birth defects in liveborn babies is 2–4% at birth. No prenatal screen or diagnostic test that is currently available can identify all of this risk. Available prenatal screening and diagnostic tests use ultrasound, maternal blood, amniotic fluid, chorionic villi, or fetal blood. Each test has specific indications and risks (Table 1). Nevertheless, even with the present technological modalities, there is no definitive genetic test to assess for all possible genetic conditions or birth defects.
TABLE 1. Prenatal tests with biological sampling
|
|||
|
Tests
|
Indications
|
Risks
|
Maternal
serum
|
Alpha-fetoprotein
(AFP)
|
Screening
test for open neural tube defects, abdominal wall defects
|
Minimal,
bruising, pain at site of blood withdrawal
|
Multiple-analyte screens
|
Screening
test for some aneuploidies including trisomy 21 (Down syndrome), trisomy 18,
|
||
Maternal
blood
|
Cell-free
fetal DNA
|
Screening
test for some aneuplodies (trisomy 13, 18, 21, sex
chromosome aneuploidy)
|
Minimal,
bruising, pain at site of blood withdrawal
|
Amniotic
fluid (amniocentesis)
|
Karyotype,
AFP DNA, enzyme, hormone analysis
|
Abnormal
screening tests,
risk of specific genetic disorder based on
personal or family history
|
≤1:500 risk for miscarriage
|
Chorionic
villi (CVS)
|
Karyotype,
DNA, enzyme, hormone analysis
|
Abnormal
screening tests,
risk of specific genetic disorder based on
personal or family history
|
≤1:400 risk for miscarriage
|
Fetal
blood (fetal blood sampling)
|
Karyotype,
DNA testing
|
Abnormal
screening tests,
risk of specific genetic disorder based on
personal or family history
|
1
to 3 in 100 risk for fetal loss
|
Ultrasound
Ultrasound, also called sonography, uses the reflection of sound waves to make an image of tissue-interfaces. These images can be highly detailed, almost photographic depictions of the embryo and fetus. Ultrasound can confirm a live pregnancy, establish gestational age, and identify twins and other multiple gestations. Ultrasound can also detect fetal abnormalities and is often the only useful prenatal test following a potential or known teratogenic exposure. While ultrasound is useful for evaluating fetal growth and development, it cannot determine the underlying cause of an abnormality or provide much information about neurological functioning of the fetus.
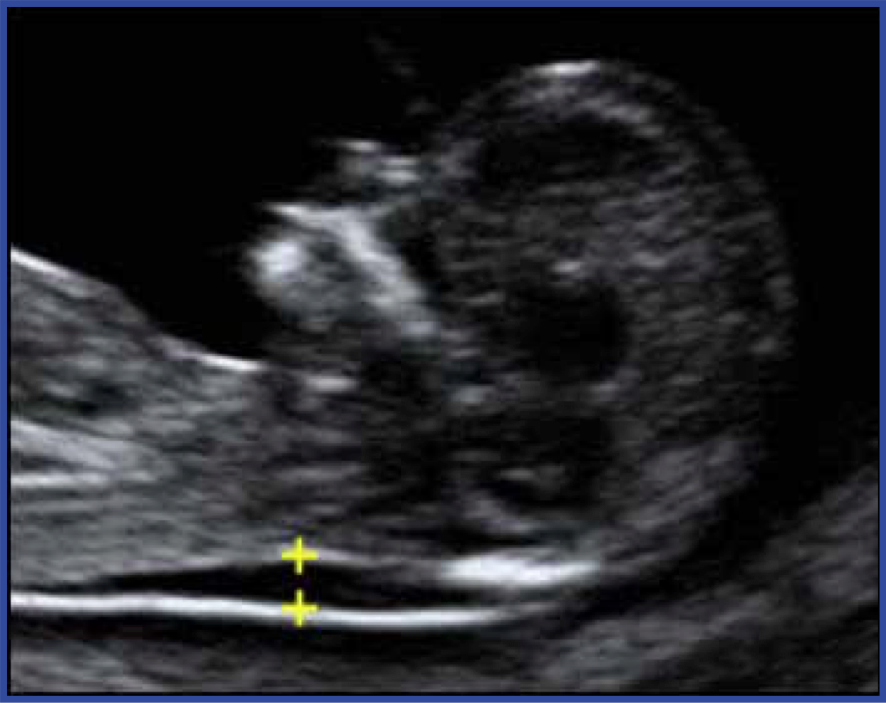
Ultrasound is used for both screening and diagnosis. For example, during the first trimester, thickening of a fluid compartment in the embryo’s neck is associated with an increased risk of certain chromosome abnormalities (Figure 1). The test is called nuchal translucency and is not diagnostic, because some embryos with increased neck fluid are normal. If ultrasound images show increased nuchal translucency, this abnormal screening test can be followed by additional testing for chromosome abnormalities (discussed below). In other cases, ultrasound can be diagnostic. For example, the accuracy of ultrasound in detecting anencephaly (incomplete head development) is approximately 100%.
It is common in the U.S. for pregnant women to have at least one or two ultrasound examinations. The first scan is performed in the first trimester to confirm gestational age and the number of fetuses. This first scan can also measure nuchal translucency and can identify some malformations and risk of chromosomal aberrations. A second ultrasound examination is performed at 18–20 weeks gestation. This ultrasound includes an anatomic survey that can identify about half of structural malformations or soft markers that could be normal variants but represent an increased risk of a chromosome abnormality, especially Trisomy 21. Sometimes the second ultrasound will be followed by a more detailed examination at a later gestational age to further define suspected structural abnormalities or to evaluate structures that were not optimally visualized during the earlier scan. For example, fetal echocardiography, a specialized ultrasound examination of the fetal heart, can be used to further characterize heart abnormalities with high accuracy.
Maternal serum screening
Maternal serum alpha-fetoprotein (MSAFP) measurement at 14–23 weeks gestation can be used to determine if the fetus is at risk for an open neural tube defect, the most common of which is spina bifida. Alpha-fetoprotein (AFP) is secreted by the fetal liver and excreted in the fetal urine, but some AFP crosses the placenta and can be measured in maternal serum. The median values of AFP in amniotic fluid and maternal serum change with gestational age, so results are expressed as multiples of the median, MoM.
Elevated MSAFP due to an open neural tube defect or abdominal wall defect can cause excessive AFP in the amniotic fluid, or an abnormal maternal-placental interface could allow excessive AFP to cross into the maternal circulation. Because AFP rises throughout pregnancy, inaccurate gestational age could cause an MSAFP level to seem high. Multiple gestations can also increase MSAFP, because more than one fetus is generating AFP. A less common reason for elevated amniotic fluid and maternal serum AFP levels are some rare inherited renal and skin diseases. Even if no reason can be found for an elevated MSAFP, the pregnancy would be considered at increased risk for preterm delivery, preeclampsia, stillbirth, or other adverse pregnancy outcomes.
A combination of analytes can be used for first- or second-trimester screening. The first-trimester screen includes the measurement of nuchal translucency with the measurement of serum analytes. First –trimester screening is performed at 11–13 weeks after the last menstrual period and second-trimester screening, which includes AFP measurement, is performed around the same time as the AFP test and is used to evaluate NTD risk. Establishing gestational age is critical for accurate interpretation because the medians for each marker change by the week. Results are adjusted for maternal age. Remember that these tests are for screening: they give rise to a risk estimate, not a definitive diagnosis. For example, a 35-year-old woman can be told that based on her age, her risk of having a fetus with aneuploidy (an abnormal number of chromosomes) such as trisomy 21 (Down syndrome) in a liveborn child is about 1 in 192. With the use of first- or second-trimester screening, that risk estimate might be altered to 1 in 10,000, 1 in 20, or something in between.
Cell-free fetal DNA
In every pregnancy, some placental DNA that is similar to the fetal DNA circulates in maternal blood. The free DNA in the mother’s blood is derived from her own cells as well as from the placenta. Quantification of DNA using nucleic acid amplification or other molecular techniques can be used to identify excess amounts (or deficits) of targeted chromosomes, giving rise to another kind of testing for aneuploidy. At present, cell-free DNA is a screening test for trisomy 13, 18, and 21 and for sex chromosome aneuploidies. Some laboratories include screening for small deletions on other chromosomes with a lower predictive value. Cell-free DNA testing is a screening test the predictive value of which depends on the age of the mother. For example, a cell-free DNA result suggesting trisomy 21 in a 17-year-old woman is more likely to be incorrect than the same result in a 40-year-old woman. It is recommended that diagnostic testing be considered following all abnormal cell-free DNA results, considering that although its detection rate for aneuploidy is very high, it is still a screening test.
Diagnostic Testing
Four different procedures can diagnose chromosomal abnormalities:
- Preimplantation embryo biopsy. The earliest test that can be done is biopsy of the early conceptus after in vitro fertilization (IVF). A fertilized egg cleaves to produce successively smaller cells one or more of which can be removed for analysis at day 3 or 5. This technique is sometimes used in couples at risk for having a child with a serious genetic disorder such as cystic fibrosis. Nucleic acid amplification such as polymerase chain reaction (PCR) and molecular techniques including chromosomal microarray and gene sequencing can be used to evaluate specific genes from a single embryonic cell to predict whether the embryo will be affected.
- Chorionic villus sampling (CVS) is performed between 10 and 12 weeks after the last menstrual period. CVS involves suctioning bits of placental tissue, called chorionic villi, through a needle or a thin tube. These bits of placental tissue usually have the same chromosome make-up as the embryo. The cells from the chorionic villi are grown in culture and their chromosomal complement is analyzed. CVS is performed under ultrasound guidance; samples can be obtained either through the cervix or through the abdomen, depending on the operator preference and location of the placenta in the uterus. The risk of miscarriage is generally described as 1/400, but is considerably lower with more experienced operators.
- Amniocentesis, the sampling of amniotic fluid, is performed in the second trimester; chromosomal analysis is performed on cells that originated in the fetal skin and have the same chromosomes as the rest of the fetus. AFP concentrations in amniotic fluid are used to test for open neural tube and a few other kinds of defects. Amniocentesis performed under continuous ultrasound guidance is described to patients as having an approximate 1/500 risk for miscarriage, although the risk with experienced operators is lower.
- Fetal blood sampling involves taking a blood sample directly from the fetus after 18 weeks of gestation. This procedure involves the removal of blood from the umbilical vein, preferably close to the placental insertion site, and is associated with a 1–3% risk of fetal loss. Sampling of fetal blood for chromosome or genetic analysis may provide a more rapid answer to specific questions if a risk has been identified based on family history, parent testing, or results of other tests, but other test methods are generally preferred due to less challenging technical requirements.
Are These Tests Useful?
The usefulness of a test depends on what you want the test to tell you. Prenatal screening tests are limited in terms of the conditions detected and cannot be expected to give yes or no answers; they simply identify a population at greater than average risk of a given disorder. For example, chromosome abnormalities occur in about 1 in 1000 pregnancies in the general population. A woman with a first trimester screen result showing a 1 in 250 chance of an affected pregnancy has a higher than average risk and she may choose to have diagnostic testing. Notice, however, that the screening test should not be interpreted as normal or abnormal result. After all, 99.6% (249/250) of women with this “abnormal” result will have an unaffected pregnancy.
Diagnostic testing, while it gives a Normal/Abnormal result, is limited to chromosome analysis, AFP testing and sometimes specific genetic conditions. A normal result does not guarantee a perfect baby, because many of the malformations that can affect fetuses are not evaluated by these tests. For example, a fetus with a ventricular septal defect will almost always have a normal result on CVS or amniocentesis testing of chromosome number. Even a relatively common genetic disorder such as cystic fibrosis will not be detected by diagnostic tests unless the cells that are obtained are specifically evaluated for the presence of a cystic fibrosis gene mutation.
Detection of an anomaly gives a pregnant patient and her partner the option to continue or terminate the pregnancy, but apart for some neural tube defects and specific cardiac abnormalities it is unusual that a detected abnormality can be repaired prior to birth. When a decision is made to continue the pregnancy, medical management may be altered, and fetal surgery is sometimes possible for certain structural anomalies. Knowing about a problem in advance also may be helpful to the family in planning to deliver at a facility where the necessary services for the baby are available. The good news is that most testing produces normal results, which can permit a couple to relax and enjoy their pregnancy if they have been concerned about abnormal outcomes.
All pregnant patients, regardless of age, history, and risk factors for chromosomal abnormalities receive counseling about available genetic screening and diagnostic tests, from no screening to an early diagnostic procedure. The decision to obtain a test is based on the patient’s (or the couple’s) desire for information, beliefs, and values.
Suggested Reading
ACOG Practice Bulletin. Neural tube defects. Obstet Gynecol
2003; 44:517–527.
ACOG Practice Bulletin. Screening for fetal chromosome abnormalities. Obstet Gynecol 2007;109:217–228.
ACOG Practice Bulletin. Ultrasonography in pregnancy. Obstetrics and Gynecology 2009; 113:451–461.
Anderson CL, Brown CE. Fetal chromosomal abnormalities: antenatal screening and diagnosis. Am Fam Physician. 2009 an 15;79(2):117–23.
Canadian Collaborative CVS-Amniocentesis Clinical Trial Group: Multicentre randomized clinical trial of chorion villus sampling and amniocentesis. Lancet 1989; 1:1.
Wald NJ, Watt HC, Hackshaw AK. Integrated screening for Down’s syndrome based on tests performed during the first and second trimesters. N Engl J Med 1999; 341(7):461–467
