About
Birth Defects Insights Blog
Birth Defects Insights is a Society for Birth Defects Research and Prevention (BDRP) blog series that provides peer-to-peer insights into cutting-edge research on birth defects and other developmental disorders and other concerns of relevance. This blog series may also be found on our Medium Channel. Members who would like to author a future blog are encouraged to contact HQ@bdrp.org.
Blogs published to date:
2025
By Melissa J. Beck, PhD
Published February 2025
This and other Birth Defects Insights blogs are available on our Medium.com channel.

On February 6, 2017, I first learned of the impending birth of my second daughter. That was the day that I also learned that she had already been exposed to a variety of drugs prenatally, and her birth mother had not had access to prenatal care for most of the pregnancy. Being a developmental toxicologist and motivated to become a second-time parent, I was not put off by this information. I was given some basic information about potential exposures but was warned that this may not be fully accurate.
Less than two months later, my daughter was born. On her first day of life, lab tests confirmed that her birthmother had continued to abuse several drugs throughout the remainder of her pregnancy, including heroin. Her birthmother volunteered that she had taken a final dose of heroin shortly before arriving at the hospital in an attempt to alleviate labor pains. This would have been my daughter's last exposure to the opioid before birth.
The amazing team of healthcare professionals immediately began to monitor her for symptoms of withdrawal over the next few days. As this was all happening during the early explosion of the opioid epidemic, the healthcare team were well-versed in diagnostic protocols. Within 24 hours of birth, she began to develop classic symptoms of neonatal abstinence syndrome (NAS), including irritability, mild tremors and muscle rigidity, among other things. Over the next several hours, symptoms increased in number and severity until it was apparent that she would need pharmacological intervention.
At the time, there were no cross-hospital standardized treatment guidelines. Healthcare professionals within each hospital developed their own approaches, and they may have varied widely from hospital to hospital and state to state. We were at a large institution in California which used methadone titrated up to alleviate symptoms of withdrawal. Their approach also involved beginning weaning off the methadone as soon as possible, and once it was demonstrated that the child could successfully handle the tapering process, the child was allowed to be discharged under the care of a pediatrician affiliated with the hospital. Traveling back to our home state of Ohio became a bit of an issue for us, since Ohio did not allow infants on methadone to be cared for at home (a tale for another time).
When I was first informed about the true nature of my daughter's exposures in the hospital, I turned to my colleagues for data. Having been a member of the Society for Birth Defects Research and Prevention (BDRP) for nearly 20 years at that time, I immediately started mining the literature. My first stop was the MotherToBaby website (being old school, I initially searched for the March of Dimes website and was appropriately directed to mothertobaby.org). The information I found there became a lifeline of hope and a trigger to dive deeper. While I had previously been aware of how gifted the members of our organization and the members of the Organization of Teratology Information Specialists, which provides the MotherToBaby service, I had never observed those giftings through the lens of a mother needing information.
After that initial review, I began performing my own literature searches, finding articles on diagnosis using Finnegan scoring (I may have begun stalking the scoring tool used by the nurses to log my daughter's symptoms), anticipated symptoms and approaches to treatment. It was at that point that I realized how varied the potential treatment plans were. I was also struck by how much we didn't (and still don't) know. For example, the immediate effects of prenatal exposure to synthetic opioids were fairly well characterized. We could expect, and did, see tremors, muscle rigidity, excessive sneezing, excessive yawning, skin mottling and skin excoriation. We were fortunate, in that most of her symptoms were not severe and that she adapted quickly to treatment. However, what became abundantly clear was the paucity of data on long-term outcomes in children who fell under her situation.
In 2018, I was fortunate to be able to attend a platform symposium on the impact of opiates on pregnancy and child development at the BDRP Annual Meeting, which was co-sponsored by the Developmental Neurotoxicity Society (DNTS). Between that symposium and my own personal research over the next months to years, I noted that much of the clinical research into long-term effects of prenatal opiate exposure was focused heavily on children who remained with their birthmothers. This made perfect sense since many children remain with their birth families. Understanding the impact of that environment on growth and development of the child would help establish family-centered goals to improve the health of the entire family and prevent some of the negative long-term outcomes that were being identified. Some of these studies have identified cognitive delays, an increased incidence of attention-deficit hyperactivity disorder and other behavioral changes in children prenatally exposed to opiates. Further research indicated that when the family was offered support, some of these outcomes were less severe.
However, there have been fewer studies that have evaluated the long-term outcomes of prenatal opiate exposure in children who were removed from the birth family altogether. Were these children as likely to exhibit the same long-term effects as noted for children remaining with their birth families? More recent studies have tried to tease out potential differences by comparing children remaining with their birth families to those that were moved to foster families, although the data from these studies are difficult to interpret for one very important reason: confounders.
Over the past nearly 8 years, I have spent more and more time following the research, trying to anticipate what we may be looking at in terms of my daughter's future healthcare needs. Yet, what has become increasingly evident is that most women who abuse drugs during pregnancy do not limit their intake to only one drug, either by choice or by expediency. Therefore, studies examining the impact of prenatal drug exposure, including those focusing on prenatal opiate exposure, are conducted in children exposed to multiple, sometimes unidentifiable, drugs. Thus, children are often grouped into those exposed to opiates with the caveat that other drugs may have been ingested during pregnancy. Alternatively, the exact exposures may be much less well-defined, such as “drug exposure,” or “multiple drug exposure,” or even “primarily opiates,” indicating that other drugs were clearly present at some point during pregnancy. Thus, the effects that have been identified may or may not be due to opiate exposure. Indeed, even our own experience bears this out, since my daughter's laboratory results indicated more than one drug was present at birth. As a scientist, I understand this lack of clarity, but as a mother, it can be enough to keep you awake at night.
As I watch my daughter grow and mature, I am reminded of the fact that she has beaten so many odds. She has been fortunate to have avoided many of the more negative consequences of prenatal opioid exposure, although she does exhibit at least one of the apparent long-term outcomes associated with the drug. However, I am also keenly aware of how much we still don't know about the extent of possible future outcomes she may face or those that are already present but as yet unidentified.
As I reflect on the fact that January is Birth Defects Awareness Month, I cannot begin to describe the pride I feel in the efforts of my colleagues across the globe who have striven to characterize, treat and prevent birth defects. I also cannot fully express my gratitude for your tireless efforts to provide hope and support for the families affected by these diseases through prevention, diagnosis, awareness of the potential outcomes and treatment. From one BDRP (family) member to another, thank you!

About the Author
Melissa J. Beck is an Associate Professor of Pharmaceutical Sciences at Cedarville University in Cedarville, Ohio. She has been a member of the Society for Birth Defects Research and Prevention since 2004 and is currently the Society's Communications Coordination Committee Chair. Melissa will be co-chairing a symposium in the Society's upcoming annual meeting titled The Ripple Effect: Neonatal Abstinence Syndrome from Birth to Adolescence.
About the Society for Birth Defects Research and Prevention
The Birth Defects Insights Blog series is published by the Society for Birth Defects Research and Prevention (BDRP). BDRP is a multidisciplinary society of researchers, clinicians, epidemiologists, and public health professionals from academia, government, and industry who study birth defects, reproduction, and disorders of developmental origin. Each summer, BDRP convenes a robust scientific meeting where members and others share their research, gain new knowledge and continuing education, mentor the next generation of researchers in the field, and network. This next annual meeting will take place June 28-July 2, 2025, in Denver, Colorado. The Society publishes the peer-reviewed scientific journal, Birth Defects Research. Learn more about BDRP at http://www.birthdefectsresearch.org and on LinkedIn.
2024
By Vijaya Kancherla, PhD, Chair, Public Affairs Committee, Society for Birth Defects Research and Prevention
Published February 2024
A companion webinar discussing the way forward will take place May 2, 2024.
For more information or to register, click here.
This and other Birth Defects Insights blogs are available on our Medium.com channel.

On May 29th, 2023, at the 76th World Health Assembly (WHA), member nations unanimously adopted the resolution entitled: Accelerating efforts for preventing micronutrient deficiencies and their consequences, including spina bifida and other neural tube defects, through safe and effective food fortification. This resolution comes thirteen years after the 63rd WHA resolution which provided recommendations on birth defects surveillance, research, prevention, and clinical care aspects to WHA member nations. Both resolutions have brought attention to birth defects globally. Through the WHA resolutions, countries are made aware of the issues related to birth defects, and recommendations are provided on addressing inequities and gaps in surveillance, research, and prevention, while improving the clinical care and quality of life of those affected, no matter where one is born or resides.
The Society of Birth Defects Research and Prevention (BDRP) published a resolution ten years ago supporting mandatory fortification of staple foods with folic acid and recommendations aiming to achieve global total prevention of folate-sensitive spina bifida and anencephaly globally by the year 2024. On this 10th anniversary of the Society’s resolution, the goal has not been achieved yet. Another global push for the cause comes from the 76th WHA resolution.
The success of the 76th WHA resolution on food fortification with micronutrients was a direct result of well-organized and strategic advocacy by multiple organizations invested in global nutrition and maternal and child health. Typically, WHA resolutions can take many years to be introduced and passed by the assembly. However, the food fortification resolution was passed in just two years. The push behind this success is the powerful partnership between academia, the medical community, patient advocacy groups, governmental and non-governmental organizations, civil society organizations, professional scientific societies, and policy makers. This blog presents a case study and the roadmap for how a WHA resolution related to birth defects prevention came to be adopted, and how partnerships worked effectively to build the momentum and reach the goal in a short span of time.
The Global Alliance for Prevention of Spina Bifida-F (Folic acid-preventable spina bifida) (GAPSBiF) was seeded with a conversation over tacos in Austin, Texas in 2015. Dr. Godfrey Oakley (BDRP member and Director of the Center for Spina Bifida Prevention at Emory University), and two neurosurgeons, Dr. Jeff Blount (Children’s of Alabama in Birmingham) and Dr. Bermans Iskandar (University of Wisconsin), connected in Austin while attending a research conference. Dr. Oakley invited Dr. Blount to lunch and discussed the willingness of pediatric neurosurgeons becoming leaders in science-based advocacy for food fortification with folic acid to prevent neural tube defects associated with maternal folate insufficiency. Passionate about preventing birth defects, they continued discussions on building a larger partnership to make an impact. The first set of partners consisted of the Center for Spina Bifida Prevention at Emory and a group of neurosurgeons, led by Drs. Jeffrey Blount and Gail Rosseau (George Washington University). Neurosurgeons perform the life-saving spinal closure surgery for spina bifida as soon as the baby is born, so they see the condition and its serious impact on the baby and family up close. With the knowledge that food fortification with folic acid effectively prevents many cases of spina bifida, and that there are thousands of preventable spina bifida cases around the world, GAPSBiF initiated its work for primary prevention with great zeal. Also, many countries where there is a high prevalence of spina bifida lack enough neurosurgeons to treat spina bifida cases promptly after birth, which leads to death of the baby or life-long health complications and disability. These neurosurgeons deeply valued primary prevention where possible as a solution. With BDRP members Dr. Godfrey Oakley and me, Vijaya Kancherla (birth defects epidemiologist and faculty at Emory University Rollins School of Public Health), the neurosurgeon team initiated strategizing prevention efforts, and founded GAPSBiF in 2019.
Drs. Blount and Rosseau, through their leadership, expanded GAPSBiF by bringing together clinical, nutrition, and public health communities. The group grew over the next few months, opening the opportunity for many stakeholders to join the advocacy effort, largely conducted through bi-weekly Zoom meetings. The number of national and international members soon exceeded the expectation, reaching over 100. The Zoom meetings included clinicians not only from neurosurgery, but outside the field (e.g., pediatricians, urologists, general surgeons), patient organizations (International Federation for Spina Bifida and Hydrocephalus), epidemiologists, health policy and public health professionals, civil society, nutrition organizations, and public health students. Meetings lasted for an hour discussing actionable plans, and everyone went to work on the cause until the next meeting. There was a deep interest, a sense of purpose, and each member had a role to play.
The partners showcased many complementary strengths and voices, all powerful in successful advocacy efforts with a shared goal to accelerate the slow pace of prevention of spina bifida worldwide. Among the first steps taken to reach the goal was the July 2022 publication of a paper in The Lancet Global Health, to reach policy makers and influential change agents. This paper made an urgent call to action for food fortification with folic acid to prevent birth defects, save lives, and promote health equity. Partners worked tirelessly and penned many other simultaneous call to actions using different avenues, including a resolution by the International Society for Pediatric Neurosurgery, helping create the needed momentum; first, for introducing the resolution at the 75th WHA in May 2022, and then, for passing it in May 2023 at the 76th WHA – a record short span of one year. The Global Alliance for Surgical, Obstetric, Trauma and Anesthesia Care (G4 Alliance), an organization with previous experience in passing other successful WHA resolutions, played a key role in bringing advocates and policy makers under one roof, and providing a fertile ground for discussions about the food fortification resolution. The G4 Alliance and partners shared important experiential learnings. Another key voice and strength to the effort came from the International Federation for Spina Bifida and Hydrocephalus (IFSBH), the representative organization of people with spina bifida and hydrocephalus and their families worldwide. For over 45 years, IFSBH worked together with its members and partners from the public, civil and private sectors to advance actions on safe and effective food fortification. In addition to the global efforts and network, local chapters of IFSBH met with policy makers in their respective countries to advocate for the upcoming resolution at the WHA.

GAPSBiF and G4 Alliance partners, including Vijaya Kancherla, participating in “Walk the Talk” event, promoting the food fortification resolution, at the World Health Assembly, Geneva, Switzerland, May 2022

World Health Assembly side-event by G4 Alliance, with partners, including Vijaya Kancherla, promoting the food fortification resolution for prevention of neural tube defects, Geneva, Switzerland, May 2022
The Colombian Ministries of Health and Foreign Affairs were major partners, and the engine for this effort at the WHA. Colombia not only sponsored the food fortification resolution at the May 2022 WHA, but also helped in developing the content to prepare its successful introduction at the WHA, and garnering support from 37 other member states (including Australia, Brazil, Canada, Chile, Ecuador, the European Union and its 27 member states, Guatemala, Israel, Malaysia, Paraguay, and the USA). Dr. Kemel Ghotme, a neurosurgeon from Colombia, and a member of GAPSBiF, served as a strong leader and advocate, pursuing every step of the resolution sponsorship by the Ministry of Colombia, and ensuring that it did not stall moving through various stages of the process. Regular consultations with the World Health Organization helped in drafting the text of the resolution. Concurrently, many individuals invested time and effort in promoting the draft resolution when it came for vote and bringing country champions onboard to support the process. This knowledge exchange and science diplomacy effort utilized top-down and bottom-up approaches, with partners from multiple disciplines, and corners of the world, contributing enthusiastically. Many NGOs and civil society organizations worked with Colombia to promote the resolution. Partners used their means and contacts to reach voting member country delegates all over the world, so the delegates were informed and voted favorably when the resolution came up for vote in WHA in May 2023.
The WHA resolution’s success also highlights a story of effective communication and coordination of multiple partners from public, private, and civic sectors. Various events were held ahead of the WHA vote as soon as the WHO Executive Board accepted the draft resolution in January 2023. Large organizations such as the Global Alliance for Improved Nutrition, Micronutrient Forum, Nutrition International, and Food Fortification Initiative, International Federation for Spina Bifida and Hydrocephalus, joined hands and were crucial partners. They formed multi-disciplinary teams and used international conferences to share the message. Every relevant opportunity was used to share about the resolution’s importance.
One such conference was the International Maternal Newborn Health Conference (IMNHC), held in South Africa, in March 2023. It was important to bring birth defects to the stage at this large international conference attended by governments, NGOs, civil society organizations, and other influential global leaders working on maternal and child health issues. A panel session was allocated at this conference, and a multi-disciplinary team of partners, including myself, could share about the upcoming resolution with attendees and delegates from over 95 countries. We could speak directly to Ministry delegates from most African and Asian countries. It was a timely conference, just a few months prior to the 2023 WHA, where the resolution would be brought to vote in Geneva, Switzerland. Another example of an event leading up to the WHA resolution was a large side-event conducted on May 23rd, 2023, in Geneva, Switzerland, right before the voting day at the WHA. This WHA side-event, titled "Fortifying the Future", further strengthened the advocacy effort by receiving multi-sectorial support from member states attending the WHA. Attendees understood the potential beneficial impact of food fortification, and its positive effect on the prevention of spina bifida and other birth defects. The benefits were not only viewed from the public health and clinical angles, but also from children’s disability rights and patients’ rights perspectives. All these efforts bore fruit on May 29th, 2023, as the member nations unanimously adopted the resolution. The WHA proceedings were relayed live on the web, and many GAPSBiF partners, including myself, who could not be in Geneva in person, anxiously followed the voting period, and cheered from distance as the resolution was passed.

"Fortifying the Future," a side-event at the WHA, with partners promoting the adoption of the resolution on food fortification for prevention of neural tube defects and micronutrient deficiencies, Geneva, Switzerland, May 2023
It has been a several months now since the fortification resolution was passed by the WHA. While all the partners celebrated the resolution’s passage, the work has only begun. The next steps are to retain the interest shown by member nations to fortify staple foods with folic acid when they voted in favor of the resolution and push for policy for implementation of fortification in over 100 countries. Time is of the essence, as waiting and inaction means preventable cases of spina bifida continue to occur in most vulnerable populations. The partnerships that developed in 2019 and worked efficiently during the pandemic years to see the resolution pass, are still active and decisive in moving the fortification implementation agenda in countries worldwide. The direct impact of this effort and success is only measurable when the world averts every possible preventable case of spina bifida and other neural tube defects.
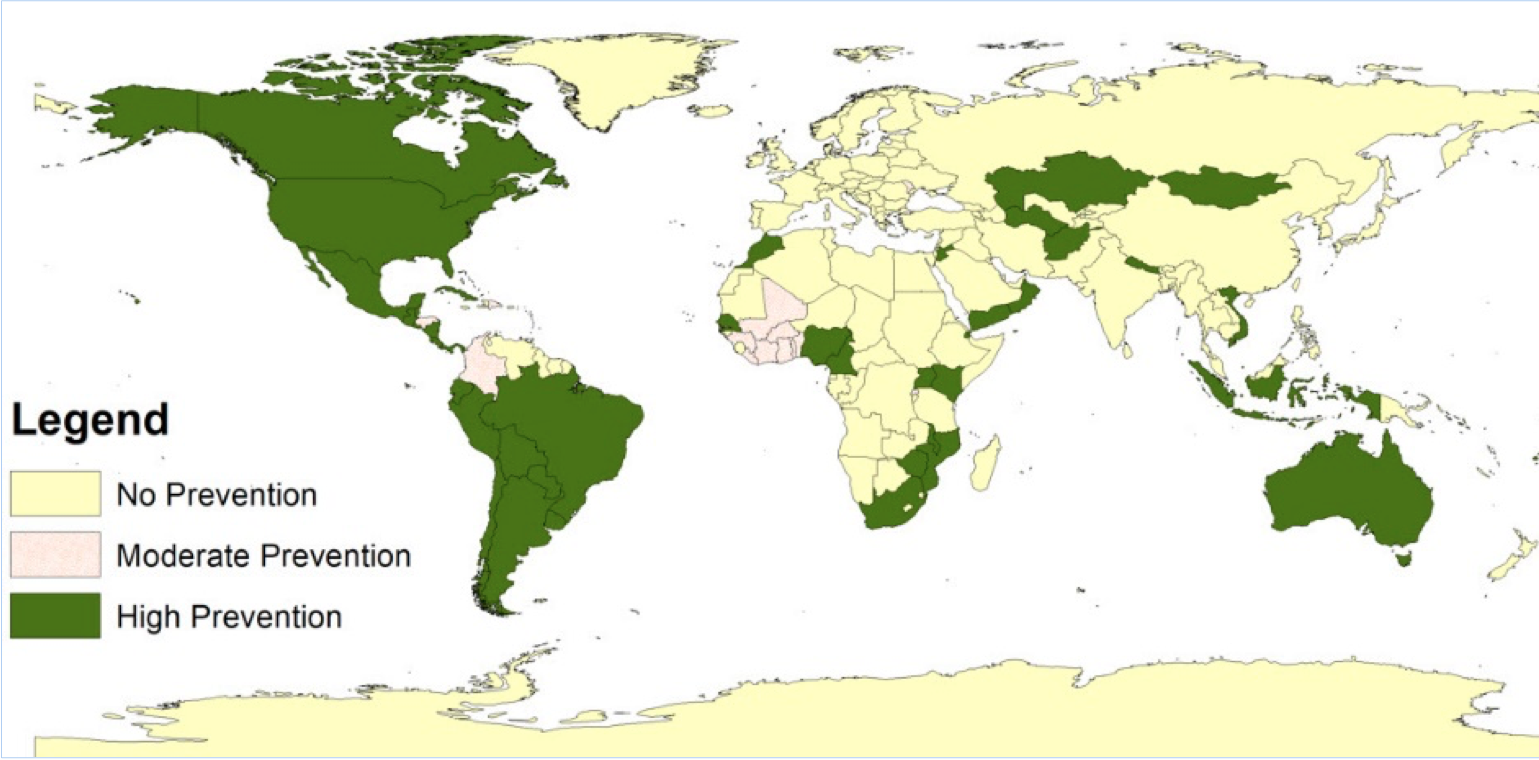
Neural tube defects prevention in countries through mandatory fortification policy of wheat flour, maize flour, and/or rice with folic acid in the year 2020. Over 100 countries, mostly in Asia, Africa, and Europe, are yet to implement food fortification program to prevent neural tube defects globally. Reference: Kancherla et al. (2022) Birth Defects Research;114(20):1392–1403
March 3rd is the World Birth Defects Day. Many organizations are partnering to use their platforms to raise awareness for birth defects. One of the highlights of this year’s World Birth Defects Day promotion is to make the WHA resolution on food fortification a focus and use opportunities that serve as a reminder to countries to implement large-scale food fortification of staples with folic acid, and any other complementary programs (e.g., folic acid supplementation) recommended in the resolution to improve micronutrient status among women of reproductive age. Several BDRP members have directly and indirectly contributed to the resolution through their participation and research. On March 3rd, we pause to reflect on the journey, celebrate these partnerships and successes, and renew global commitment to action on preventing birth defects where possible, while also improving care for those living with these conditions.
A companion webinar discussing the way forward will take place May 2, 2024. For more information or to register, click here.
This and other blogs in the Birth Defects Insights blog series may be found on BDRP’s Medium Channel.

About the Author
Vijaya Kancherla, PhD, is an Associate Professor in the Department of Epidemiology at Emory University Rollins School of Public Health. She also serves as the Deputy Director of the Center for Spina Bifida Prevention at Emory University. Vijaya is the current Chair of the Public Affairs Committee of the Society for Birth Defects Research and Prevention. Her research is focused on birth defects epidemiology, covering topics such as surveillance, etiology, prevention, health care outcomes, and policy, both in the U.S. and internationally.
About the Society for Birth Defects Research and Prevention
Healthy pregnancies. Healthy babies. Better lives.
The mission of the Society for Birth Defects and Prevention (BDRP) is to understand the cause and pathogenesis of disorders of developmental and reproductive origin to prevent their occurrence and improve outcomes through research, collaboration, communication, and education.
BDRP is a multidisciplinary society of scientists from a variety of disciplines including researchers, clinicians, epidemiologists, and public health professionals from academia, government, and industry who study birth defects, reproduction, and disorders of developmental origin. Our members include those specializing in cell and molecular biology, developmental biology and toxicology, reproduction and endocrinology, epidemiology, nutritional biochemistry, and genetics, as well as the clinical disciplines of prenatal medicine, pediatrics, obstetrics, neonatology, medical genetics, and teratogen risk counselling.
BDRP convenes an annual scientific meeting annually where members and others share their research, gain new knowledge and continuing education, mentor the next generation of researchers in the field, and network. The Society publishes the peer-reviewed scientific journal, Birth Defects Research. Learn more at http://www.birthdefectsresearch.org. Find BDRP on LinkedIn, Facebook, Twitter and YouTube.
References
Borman, E. D., Foster, W. G., Greenacre, M. K. E., Muir, C. C. and deCatanzaro, D. (2015). Stress lowers the threshold dose at which bisphenol A disrupts blastocyst implantation, in conjunction with decreased uterine closure and e-cadherin. Chemico-Biological Interactions 237, 87-95.
Carlsen, E., Giwercman, A., Keiding, N. and Skakkebaek, N. E. (1992). Evidence for decreasing quality of semen during past 50 years. BMJ 305, 609-613.
Puscheck, E. E., Awonuga, A. O., Yang, Y., Jiang, Z. and Rappolee, D. A. (2015). Molecular biology of the stress response in the early embryo and its stem cells. Adv Exp Med Biol 843, 77-128.
Puscheck, E. E., Ruden, X., Singh, A., Abdulhasan, M., Ruden, D. M., Awonuga, A. O. and Rappolee, D. A. (2022). Using high throughput screens to predict miscarriages with placental stem cells and long-term stress effects with embryonic stem cells. Birth Defects Res.
Steptoe, P. C. and Edwards, R. G. (1978). Birth after the reimplantation of a human embryo. Lancet 2, 366.
Wilcox, A. J., Weinberg, C. R., O'Connor, J. F., Baird, D. D., Schlatterer, J. P., Canfield, R. E., Armstrong, E. G. and Nisula, B. C. (1988). Incidence of Early Loss of Pregnancy. New England Journal of Medicine 319, 189-194.
2023
By Daniel Rappolee, PhD
Published June 2023
This and other Birth Defects Insights blogs are available on our Medium.com channel.

I have two brothers and I am the middle child. My mother suffered a miscarriage between me and my younger brother, which she attributed to heavy fumes from lead-based paints at a new house shortly before the miscarriage. Many women have suffered through miscarriage, and speculations on causes are called “anecdotes” in science. But science is systematic and requires larger epidemiological associations between hypothetical causes and events of miscarriage. These data are remarkably hard to get.
But there is a persistent problem of obtaining funding and getting miscarriage data. Despite poor funding, the scope of the issue is immense. The Fertility and Infertility Branch of the National Institute of Child Health and Development (NICHD) recently posted that miscarriage occurs in 70% of fertilized embryos and their Strategic plan #2 is studying miscarriage mechanisms (https://www.nichd.nih.gov/about/org/der/branches/fib).
This gap of knowledge should be compared with our knowledge of sperm, where number and quality have been quantified for nearly a century. The first human sperm counts in the US were recorded in 1934, and 50-year reviews occurred 30 years ago (Carlsen et al., 1992). The conclusions were that sperm counts have dropped precipitously, from 113 to 66 million/small volume, suggesting environmental influence. In contrast, access to human embryos and oocytes is very low, but screening toxicant effects on pluripotent stem cells acting as proxies for embryos can predict miscarriage in humans.
Have miscarriage rates increased in the last 50 years and what are the environmental causes of higher miscarriage? Since the first in vitro fertilization (IVF) birth in 1978 (Steptoe and Edwards, 1978), and the production of accurate tests for early pregnancy hormones by 1988 (Wilcox et al., 1988), scientists reported early miscarriage rates. To this day most Google searches for “miscarriage” cite only a 10% miscarriage rate of later gestation fetuses that endanger maternal health and are easily detectable. Because 70% miscarriage occurs in early pregnancy, detectable only recently, questions on environmental causes or associations with miscarriages are surprisingly hard to answer.
How do we get data on oocyte and embryo number and quality? Environmental influences are being studied by zip code level measurements of environmental pollutants and their associations with miscarriage. Cell phone apps measure urban stresses telemetrically and test for their association with miscarriage. One telephone app detects loud noises associated with increased stress hormones. In animal models, cortisol slows growth of early pregnancy embryos that may contribute to miscarriage (Puscheck et al., 2015), and cortisol can sensitize rodents to environmental toxicants, increasing embryo loss.
In controlled studies in animal models like rodents, the maternal stress hormone cortisol exacerbates embryo loss caused by environmental pollutants such as BPA (Borman et al., 2015) when both stress stimuli are applied to normal females after fertilization. Thus, BPA and cortisol exacerbate loss of normal embryos. Current studies in our lab will test whether stress hormone levels in women in early pregnancy during the first trimester, when almost all miscarriages occur, exacerbate cellular changes which would lead to miscarriage induced by environmental pollutants.
Our project started over 15 years ago, with stress effects on early post-fertilization mouse embryos in IVF culture, and their component embryonic and placental trophoblast stem cells (ESC and TSC, respectively). Our lab developed an in vitro approach 8 years ago using high throughput screens (HTS) in stem cells to test for environmental and hormonal stresses that may cause miscarriage or birth defects.
During early pregnancy when most miscarriages occur, the embryo is simple. Soon after fertilization, the embryo is composed only of ESC and TSC. We use ESC and TSC with viable status reporters of stemness/differentiation/cell cycle status to report time and dose-dependent effects of stress hormones (cortisol), toxicants (phthalates, PFAS) and drugs (e.g., aspirin, retinoids) in HTS to test many single and mixed stress effects. For a small toxicant set, initial studies suggest that TSC are more sensitive and differentiate more stem cells than ESC (Puscheck et al., 2022). We will test this hypothesis on a larger set of environmental and hormonal stressors.
Understanding stress effects during pregnancy could be used to inform risk assessment decisions to make policy changes and increase pre- and postnatal health. It seems likely that embryos surviving this period of immense loss and miscarriage may survive some exposure to stress. Thus, a second important aspect of research on this development period is to understand the exposures and types of stress that do not cause embryo loss but cause stress that leads to postnatal effects. The 70% embryo loss is a massive issue to understand for environmental causes, but the 30% surviving embryos (e.g., many that developed and may be reading this blog!), are unlikely to have traversed early development without making some developmental (lineage imbalance) and epigenetic adaptations that affect health.
Birth defects, epidemiology, and associated and causal findings in animal models, is a large international research endeavor. Due to inaccessibility and the focus of funding on surviving embryos (offspring), the area of miscarriage research is remarkably small. But this is likely to change. The recent Dobbs decision overturning Roe vs. Wade may bring new focus on early embryo health. There is an interest in what may cause embryonic death and long-term pre- and postnatal effects on the health of offspring when embryos survive the miscarriage period.

About the Author
Dr Rappolee received a bachelor’s degree at University of California, Santa Barbara (UCSB), a doctorate at University of California, San Francisco (UCSF), and has been on faculty at Northwestern University and Wayne State University (WSU). Dr. Rappolee’s lab, at WSU Medical School in midtown Detroit, MI, investigates the effects of stress on early embryonic development and how this affects the efficacy of fertility treatments clinically, and miscarriage and birth defects toxicologically. His lab’s goal is to make healthier babies.
Dr. Rappolee and his lab have had many collaborators. Dr Elizabeth Puscheck and Dr. Niyi Awonuga at WSU have helped with IVF stress effects during the miscarriage period. Dr. Ali Faqi, past head of Developmental and Reproductive Toxicology (D.A.R.T). at MPI Research, introduced them to the field of Developmental Toxicology. Advice from Dr. Tom Knudsen at the Environmental Protection Agency/National Center for Toxicogenomics/National Center for Computational Toxicology (EPA/NCT/NCCT), Josh Robinson at UCSF, and Dr. Doug Ruden at WSU advanced their studies. Dr. Hao Feng at Case Western Reserve University helped with scRNAseq “stress fingerprints”.
About the Society for Birth Defects Research and Prevention
Healthy pregnancies. Healthy babies. Better lives.
The mission of the Society for Birth Defects and Prevention (BDRP) is to understand the cause and pathogenesis of disorders of developmental and reproductive origin to prevent their occurrence and improve outcomes through research, collaboration, communication, and education.
BDRP is a multidisciplinary society of scientists from a variety of disciplines including researchers, clinicians, epidemiologists, and public health professionals from academia, government, and industry who study birth defects, reproduction, and disorders of developmental origin. Our members include those specializing in cell and molecular biology, developmental biology and toxicology, reproduction and endocrinology, epidemiology, nutritional biochemistry, and genetics, as well as the clinical disciplines of prenatal medicine, pediatrics, obstetrics, neonatology, medical genetics, and teratogen risk counselling.
BDRP convenes an annual scientific meeting annually where members and others share their research, gain new knowledge and continuing education, mentor the next generation of researchers in the field, and network. The Society publishes the peer-reviewed scientific journal, Birth Defects Research. Learn more at http://www.birthdefectsresearch.org. Find BDRP on LinkedIn, Facebook, Twitter and YouTube.
References
Borman, E. D., Foster, W. G., Greenacre, M. K. E., Muir, C. C. and deCatanzaro, D. (2015). Stress lowers the threshold dose at which bisphenol A disrupts blastocyst implantation, in conjunction with decreased uterine closure and e-cadherin. Chemico-Biological Interactions 237, 87-95.
Carlsen, E., Giwercman, A., Keiding, N. and Skakkebaek, N. E. (1992). Evidence for decreasing quality of semen during past 50 years. BMJ 305, 609-613.
Puscheck, E. E., Awonuga, A. O., Yang, Y., Jiang, Z. and Rappolee, D. A. (2015). Molecular biology of the stress response in the early embryo and its stem cells. Adv Exp Med Biol 843, 77-128.
Puscheck, E. E., Ruden, X., Singh, A., Abdulhasan, M., Ruden, D. M., Awonuga, A. O. and Rappolee, D. A. (2022). Using high throughput screens to predict miscarriages with placental stem cells and long-term stress effects with embryonic stem cells. Birth Defects Res.
Steptoe, P. C. and Edwards, R. G. (1978). Birth after the reimplantation of a human embryo. Lancet 2, 366.
Wilcox, A. J., Weinberg, C. R., O'Connor, J. F., Baird, D. D., Schlatterer, J. P., Canfield, R. E., Armstrong, E. G. and Nisula, B. C. (1988). Incidence of Early Loss of Pregnancy. New England Journal of Medicine 319, 189-194.
By Mona Dai
Published April 2023
This and other Birth Defects Insights blogs are available on our Medium.com channel.

Standing outside the Russell Senate Office Building, I felt small as I took in the giant marble building. The sunshine blinded me as I stared at the intimidating yet beautiful white building that took up an entire square block by itself. It was a windy day on Wednesday, March 15th and I was in Washington D.C. for the Federation of American Societies for Experimental Biology (FASEB) Capitol Hill Day as a recipient of the Society for Birth Defects Research & Prevention (BDRP) funded FASEB Howard Garrison Public Affairs Fellowship. Despite feeling a bit nervous, I was also very excited to be able to participate in the Capitol Hill Day in person, three years after winning the award back in 2020.
After getting temporarily lost, I met up with the other members of my group: Dr. Lee-Ann Allen from the Society for Leukocyte Biology (SLB) and Chair of the Department of Molecular Microbiology & Immunology at University of Missouri (Mizzou) and our guide Meg Thompson from Federal Science Partners (FSP). Together, we would be advocating for advancing science research funding to Senator Josh Hawley and Representative Blaine Luetkemeyer, Congressional representatives from Missouri. We brainstormed a bit in the basement café what we would say in our meetings as Meg gave us advice on how to best construct our pitches.
Since Lee-Ann and I primarily relied on funding from the National Institutes of Health (NIH), we decided to focus on FASEB’s ask for Congress to dedicate at least $51 billion to NIH for the upcoming fiscal year. Lee-Ann first introduced herself and her work on how bacteria can manipulate cells to cause ulcers and cancer in humans. She explained how Missouri’s previous senator, Roy Blunt, had championed biomedical research and how his support at Mizzou had led to the opening of the Roy Blunt NextGen Precision Health building, a facility that focuses on critical health care research. I then explained that I was a PhD student focusing on environmental engineering and public health. Specifically, I research issues related to public drinking water contamination of chemicals such as arsenic and manganese (metals that have been associated with birth defects and other adverse health effects), and mapping areas in the US where their concentrations could be considered unsafe. Closer to home, we also discussed how the current water crisis in St. Charles (a county near where I grew up in St. Louis) has led to the county shutting down 6 of its 7 water wells due to contamination by Ameren Missouri, the main power utility provider in the area.
It was very gratifying to have the opportunity to speak to Senator Hawley’s and Representative Luetkemeyer’s offices. Everyone was friendly, and it felt fantastic to be able to interact with their staff who were extremely engaged in our conversation, took our concerns seriously, and understood the importance of why we flew all the way to Washington D.C. to meet with them. As an unexpected surprise, Representative Luetkemeyer’s office also gave us passes to visit the Capitol Galleries and we were able to end our day soaking in the House and Senate chambers.
Collectively, 45 scientists representing 25 societies visited D.C. on FASEB’s Capitol Hill Day, meeting with 75 House and Senate offices. Through the maze of hallways that we traversed passing well-dressed professionals in suits, huddled lobbyists discussing tactics, and casually dressed families on vacation, I always felt comforted whenever I caught sight of a FASEB badge like mine hanging on someone’s neck and knew that we were on the same team. I am extremely thankful to BDRP and FASEB for providing the opportunity to involve scientists in advocacy & lobbying. It was such a whirlwind day, yet a very meaningful experience. I am so grateful to have participated and would look forward to repeating again!

About the Author
Mona Dai is a PhD candidate in environmental science and engineering at the Harvard John A. Paulson School of Engineering and Applied Sciences. She uses data science to look at spatial patterns of sociodemographic factors and contamination of public drinking water systems in the United States.
About the Society for Birth Defects Research and Prevention
Healthy pregnancies. Healthy babies. Better lives.
The mission of the Society for Birth Defects and Prevention (BDRP) is to understand the cause and pathogenesis of disorders of developmental and reproductive origin to prevent their occurrence and improve outcomes through research, collaboration, communication, and education.
Scientists interested in or already involved in research related to topics mentioned in this blog are encouraged to join BDRP and attend the 63rd Annual Meeting taking place June 24–28, 2023, in Charleston, South Carolina.
BDRP is a multidisciplinary society of scientists from a variety of disciplines including researchers, clinicians, epidemiologists, and public health professionals from academia, government, and industry who study birth defects, reproduction, and disorders of developmental origin. Our members include those specializing in cell and molecular biology, developmental biology and toxicology, reproduction and endocrinology, epidemiology, nutritional biochemistry, and genetics, as well as the clinical disciplines of prenatal medicine, pediatrics, obstetrics, neonatology, medical genetics, and teratogen risk counselling.
BDRP publishes the peer-reviewed scientific journal, Birth Defects Research. Learn more at http://www.birthdefectsresearch.org. Find BDRP on LinkedIn, Facebook, Twitter and YouTube.
By Amanda Dorsey, BS, (MPH Candidate) and Vijaya Kancherla, PhD
Department of Epidemiology, Emory University Rollins School of Public Health
Published March 2023
This and other Birth Defects Insights blogs are available at Medium.com.

Amanda Dorsey and Vijaya Kancherla with the COE neurosurgery team at Zewditu Memorial Hospital, Addis Ababa, Ethiopia
Last summer in the pediatric neurosurgical wards of Addis Ababa, the capital city of Ethiopia and sprawling metropolis with a population of 5.2 million, we, Ms. Amanda Dorsey (MPH Candidate) and Dr. Vijaya Kancherla (Assistant Professor), from the Department of Epidemiology at Emory University, had an incredibly rich experience in the field of spina bifida research, prevention, and care. This experience sheds light on global birth defects, which we felt was beneficial to share with other BDRP members and trainees through this blog. More importantly, this experience was made possible by the life work of another Society member whom we wish to recognize and honor, the late Dr. Marinus Koning, Director of ReachAnother Foundation, a non-governmental organization invested in the clinical care and prevention of spina bifida in Ethiopia.
The project that we undertook was conducted in collaboration with ReachAnother Foundation and supported by the Emory University Rollins School of Public Health Global Field Experience Financial Award (a grant for public health graduate students to pursue at least 6 weeks of global epidemiology research in low- and middle-income countries). We were collaboratively conducting a program evaluation for ReachAnother Foundation’s care and prevention programs in several hospitals in Ethiopia, where the Foundation established multiple Centers of Excellence (COE) in pediatric neurosurgical care. At each location, the Foundation has built neurosurgical capacity to treat spina bifida and hydrocephalus patients and provide follow-up care for the children.
The prevalence of neural tube defects in Ethiopia is very high. In absolute numbers, there are about 10,500 babies born with spina bifida in Ethiopia each year. Very few make it to their fifth birthday, with a huge disparity in outcomes based on family income and the availability of surgical resources. The time window to treat a newborn with spina bifida for optimal outcomes is very short, and few are able to access surgery in a timely manner, which is exclusively available in bigger cities.
ReachAnother Foundation has been doing exceptional work in Ethiopia since 2006, training neurosurgeons to provide spina bifida closure surgeries, manage hydrocephalus, and deliver multidisciplinary care for the whole family. The Foundation also recently set up a database to document outcomes among babies who received care for spina bifida or hydrocephalus. Each of their COE works with the database, entering detailed data on spina bifida and hydrocephalus patient encounters and surgical care indicators. Additionally, they created a pediatric neurosurgery training program for nurses to contribute to the multidisciplinary care of their patients.
We had the opportunity to assist the ReachAnother Foundation by evaluating their initiatives in each COE in collaboration with local partners. We evaluated the database of one tertiary care center located in Addis Ababa, which included over 1,100 patients. Based on the available data, we, alongside local collaborators, conducted an epidemiological study on the timeliness of myelomeningocele closure surgery among spina bifida patients treated at the hospital. We interviewed the physicians and staff on the strengths and challenges of the database. We also studied folic acid prescription practices to prevent recurrence of spina bifida.
It was humbling to learn how tirelessly the healthcare professionals in the pediatric neurosurgery wards work with their patients at the forefront of their priorities. This included the neurosurgeons, general practitioners, nurses, care coordinators, and more. But still, the number of spina bifida patients in Ethiopia surpasses the country-wide neurosurgical capacity, warranting further resources and training. In addition to a big push for training more doctors and increasing surgical care, Ethiopia has recently approved mandatory folic acid fortification of wheat flour to prevent the majority of neural tube defects.
In focus group interviews with pediatric neurosurgery nurses, we learned that the nurses took great pride in encouraging mothers to take their folic acid pills to prevent a subsequent pregnancy with spina bifida. Signs and promotional materials about folic acid were posted all around the hospitals for new and pregnant mothers to see; however, there is a need to strengthen recurrence prevention programs that provide mothers who are at a high risk for having a subsequent pregnancy impacted by spina bifida with a higher dose of folic acid (4,000 mcg/day) consistently.
This was an amazing learning and mentoring experience for us, while we both also learned from our Ethiopian colleagues in a short span of 6 weeks on the ground in Ethiopia. Many lessons were learned on global health advocacy that surpass the realm of textbooks and can only be gained through thorough formative work and total immersion with close local partnerships and collaborations. There were hardworking doctors, nurses, and healthcare professionals providing unconditional care for patients and their families with whatever resources they could. Some of the nurses went to the extent of setting up a fund or donating a part of their salary to support mothers.
We are inspired by the opportunities for improvement and further research related to spina bifida care and prevention in Ethiopia. We built partnerships and friendships with Ethiopian colleagues that are fundamental to effective work in public health. The summer study turned into a rich student-mentor engagement loaded with insights on global birth defects research and prevention, fruitful collaborations with the team in Ethiopia, and many lessons on the strengths and perseverance of people involved in global birth defects work.
About the Society for Birth Defects Research and Prevention
Healthy pregnancies. Healthy babies. Better lives.
The mission of the Society for Birth Defects and Prevention (BDRP) is to understand the cause and pathogenesis of disorders of developmental and reproductive origin to prevent their occurrence and improve outcomes through research, collaboration, communication, and education.
Scientists interested in or already involved in research related to topics mentioned in this blog are encouraged to join BDRP and attend the 63rd Annual Meeting taking place June 24–28, 2023, in Charleston, South Carolina. BDRP is a multidisciplinary society of scientists from a variety of disciplines including researchers, clinicians, epidemiologists, and public health professionals from academia, government, and industry who study birth defects, reproduction, and disorders of developmental origin. Our members include those specializing in cell and molecular biology, developmental biology and toxicology, reproduction and endocrinology, epidemiology, nutritional biochemistry, and genetics, as well as the clinical disciplines of prenatal medicine, pediatrics, obstetrics, neonatology, medical genetics, and teratogen risk counselling. BDRP publishes the peer-reviewed scientific journal, Birth Defects Research. Learn more at http://www.birthdefectsresearch.org. Find BDRP on LinkedIn, Facebook, Twitter and YouTube.
Published January 2023

By Sidra Shafique, PhD
This blog is also available on our Medium Channel.
Computational Modeling and Machine Learning: The Cutting-Edge Innovative Tools for Birth Defects Research, Prevention, and Treatment
"A baby learns to crawl, walk, and then run. We are in the crawling stage when it comes to applying machine learning."
Dave Waters
Computational modeling and machine learning have applications in understanding the embryogenesis and the morphological basis of birth defects. Algorithms are created in machine learning to build a model based on a set of sample data. The model is then validated against experimental data for its accuracy and output. The disciplines most closely related to machine learning are bioinformatics, computational anatomy, computational modeling, and systems biology. All these disciplines act at the interface of mathematical, statistical, and data-analytical methods. Computational models can be used to run simulations to understand the outcomes of perturbations in genetic pathways to generate a hypothesis for testing in new experiments, to identify potential therapeutic targets, and so on.
I envision using available data in computational modeling for developmental sciences. As a researcher interested in the mechanisms of neural tube defects (NTDs), I understand that the NTDs result from failed neural tube closure during the first four weeks of embryonic development (24 days post-fertilization)1. Underpinning these defects is a series of events involving signaling pathways, cytoskeletal components, and cell- and tissue-level mechanical interactions. Computational modeling has been effectively used to create a neural tube closure model. To understand how this model works, we need to know how it was created. The neural tube closure computational model of Brodland et al. (2010) effectively investigated the mechanics of neural plate morphogenesis, the formation of neural ridges, and prospective closure. This multiscale computational model of the whole embryo was created by dividing the surface epithelium into triangular regions consisting of several tens of cells. Each of these regions is represented along the apical and basal surfaces of the monolayer tissue, thus replicating the active forces produced by its cells. Thousands of cell-level simulations were incorporated to investigate the mechanics of embryonic epithelium closure. The cell-level constitutive equations relating stress, strain, cellular fabric, lamellipodium action, and other relevant factors were constructed. Then the model was created by first modeling a single cell, then a sheet of cells, followed by modeling a whole embryo using data from images taken from 63 different angles, consisting of 1282 surface points, and 2559 triangles of a live axolotl embryo. The simulation then was successfully run in the model to understand the impacts of absent neural ridge forces, unilateral absent neural ridge forces, increased thickness of neural ridge epithelium, and so on.2
Transforming the imaging data with calculation power and algorithms into sophisticated computation models and simulation is an emerging integrative discipline. Recently Dokmegang et al. (2021) created a computational model of the epiblast and trophectoderm using MG# (MechanoGenetic Sharp), an original computational model of biomechanics. The parent MG# project enables anyone with minimal or no programming experience to run biological simulations and visualise the results. MG#Core is the computational engine of MG#. It runs simulations and logs results into (custom) MG files. Using this MG# model, it was possible to reproduce key cell shape changes and tissue level behaviors in silico, while accounting for internal, cytoskeletal, and external forces. The simulations were successfully run to understand the epiblast remodeling and position relative to the trophectoderm.3
Computational models offer a holistic approach to combining, integrating, and visualizing experimental data. We see new scientific information coming along every day. However, this scattered and patchy information needs to be processed as 'Big Data'. The discipline of bioinformatics applies network analysis to analyze and understand -omics data including genome, transcriptome, proteome, and metabolome. In most cases, the network analysis is formally focused on a defined set and source of data. Machine learning is the next step where algorithms would be used to predict the morphological outcome based on existing data. Machine learning, as a valuable visual tool, can help us understand the links between physical processes, gene expression, and cell fate. Although, it seems challenging to model the very dynamic multi-scale processes underlying embryogenesis by incorporating physics, genetics, epigenetics, and mechanics at the same time. The scientific community still needs to understand the importance of making available and maintaining open-source modeling codes. Despite this and other challenges, I believe that machine learning models created from embryonic morphogenesis and imaging data with multiscale biophysical algorithms can be used as a future clinical tool to predict birth defects. The obstetrical ultrasound and doppler scans provide us with high-resolution imaging data. For human pregnancies with a history of birth defects, patient data can be used to run simulations in already created models to evaluate the risk of prospective birth defects in the developing embryo. This information is of value from both the preventive and therapeutic perspectives. As mentioned before, we are just 'crawling' in this discipline. However, machine learning could be an effective and powerful diagnostic and therapeutic tool for human pregnancy evaluation in decades to come.
About the author

Sidra Shafique is a Postdoctoral Fellow studying developmental toxicology at Queen’s University, Canada. Her Master’s and Doctorate research focused on developmental sciences. She is a medical doctor (MBBS), Fellow (FCPS), and Member (MCPS) of the College of Physicians and Surgeons Pakistan in Obstetrics and Gynecology.
About the Society for Birth Defects Research and Prevention
Healthy pregnancies. Healthy babies. Better lives.
The mission of the Society for Birth Defects and Prevention (BDRP) is to understand the cause and pathogenesis of disorders of developmental and reproductive origin to prevent their occurrence and improve outcomes through research, collaboration, communication, and education.
Scientists interested in or already involved in research related to topics mentioned in this blog are encouraged to join BDRP and attend the 63rd Annual Meeting taking place June 24–28, 2023, in Charleston, South Carolina. BDRP is a multidisciplinary society of scientists from a variety of disciplines including researchers, clinicians, epidemiologists, and public health professionals from academia, government, and industry who study birth defects, reproduction, and disorders of developmental origin. Our members include those specializing in cell and molecular biology, developmental biology and toxicology, reproduction and endocrinology, epidemiology, nutritional biochemistry, and genetics, as well as the clinical disciplines of prenatal medicine, pediatrics, obstetrics, neonatology, medical genetics, and teratogen risk counselling. BDRP publishes the peer-reviewed scientific journal, Birth Defects Research. Learn more at http://www.birthdefectsresearch.org. Find BDRP on LinkedIn, Facebook, Twitter and YouTube.
References
1. Greene, N. D. E. & Copp, A. J. Neural tube defects. Annual Review of Neuroscience 37, 221–242 (2014).
2. Wayne Brodland, G., Chen, X., Lee, P. & Marsden, M. From genes to neural tube defects (NTDs): insights from multiscale computational modeling. HFSP Journal 4, 142 (2010).
3. Dokmegang, J., Yap, M. H., Han, L., Cavaliere, M. & Doursat, R. Computational modelling unveils how epiblast remodelling and positioning rely on trophectoderm morphogenesis during mouse implantation. PLoS ONE 16, 1–20 (2021).
2022
Published June 2022

By Michael Golding, PhD
This blog is also available on our Medium Channel.
A Father's Day question: Why do fathers (epigenetically) treat their sons and daughters differently? The frustrations and musings of an alcohol researcher working in the field of paternal epigenetic programming.
Sociological research indicates that in the United States, parents desire families with a child of each gender. However, fathers spend more time with their sons than daughters and are more likely to support male offspring in reaching their academic and professional goals (1). Therefore, consciously or not, males preferentially invest more resources in their sons.
Our efforts to understand the developmental origins of birth defects and disease have recently expanded to include paternal exposures before conception, which emerging clinical and animal model research can link to a range of physiologic and behavioral changes in the next generation. Interestingly, here as well, alterations in the paternal epigenetic program appear to preferentially impact the male offspring, albeit with predominantly negative outcomes. For the past six years, my research group has focused on examining the impact of paternal alcohol exposures on offspring health and development (2–7). Through these studies, we have consistently identified adverse outcomes in placental development and long-term health measures in male offspring but less so in females. Others observe similar results in studies examining different drugs of abuse, including cocaine and cannabis (8–10). The transmission of a paternal memory that only affects male progeny, while interesting, is vexing and highlights the complexity of mammalian mechanisms of epigenetic inheritance.
Investigation into the impacts of preconception paternal alcohol exposures on offspring health was a challenging field to enter. After all, the cause of Fetal Alcohol Spectrum Disorders (FASDs) is defined by the warning label on the bottle. Furthermore, across the CDC, the NIH, and all levels of society, the perception that FASDs are the consequence of maternal drinking is thoroughly entrenched. The challenge of questioning this existing maternal-centric paradigm lies not in devising an elegant experimental design but in attempting to overhaul the maternal-centric view of study section. Yes, I understand maternal alcohol exposures are relevant to FASDs. Yes, I know that most men are unaware of non-genetic mechanisms of inheritance or their part in embryonic programming, and realistically, male alcohol use will not decrease. In fairness, messaging from the CDC and NIH-NIAAA cannot broadly achieve this in women either. Nevertheless! Although half of all pregnancies are unplanned, many male partners are heavily engaged in family planning—especially couples struggling with fertility. Importantly, we strongly suspect male alcohol use negatively impacts in vitro fertilization pregnancy rates. Further, our published data support not only an immediate effect of alcohol on sperm but also short-term effects on embryo growth and longer-term impacts on the metabolic health of the adult offspring (2–7). Therefore, determining the impact of paternal alcohol use on offspring growth and development is essential to defining the breadth of factors influencing FASD outcomes and potentially explaining the wide variation observed in the penetrance and variation of this disorder.
Part of the hesitation to adequately consider a male contribution to FASDs lies in two core misconceptions: that toxicological outcomes are an acute response (not programmed) and that sperm only transmit DNA. First, our research demonstrates that paternal alcohol exposures induce placental abnormalities and fetal growth restriction in the offspring (2, 3, 6, 7). As none of the dams ever see a drop of alcohol, the induced outcomes we observe do not follow a classic acute toxicological paradigm. Instead, they demonstrate that some aspects of teratogenesis are programmed and exert their effects in a subsequent life stage. Second, during the late 1980s, McGrath and Solter demonstrated that the sperm and egg contain information beyond the genetic code and make unequal contributions to the developing offspring (11). Specifically, their experiments revealed that information in sperm drives the development and differentiation of the placenta, and from this work, the field of genomic imprinting was born. Today, we know that sperm carry an expansive suite of epigenetic information, including DNA methylation, posttranslational histone modifications, and noncoding RNAs. Moving forward, we must recognize that toxicological outcomes do not need to entail cell death or other acute measures but can arise from changes in epigenetic programming that impact physiology during a subsequent life stage. Further, we must consider gametes the same as any other precursor cell type and recognize that epimutations acquired during the formation of sperm are likely to have just as much of an impact on the developmental program as exposures during preimplantation development.
However, although we can identify alcohol-induced changes in sperm-inherited epigenetic marks, we do not understand why these modifications predominantly impact the male offspring. Are females simply more adaptable than males? If so, sperm-inherited epigenetic memories may represent a transient stressor to which males and females respond differently. If this is the case, is everything else we observe a symptom of this early stressor? Perhaps the answer to my question is that females adapt to the abnormalities in the paternally inherited program and avoid the observed symptoms. Indeed, there is growing evidence that differences between male and female placentation confer different growth rates during different phases of pregnancy, which could influence sexually dimorphic disease outcomes (12). Previous studies have identified significant epigenetic differences between the male and female liver that confer differing abilities to metabolize drugs and predispositions to cancer (13, 14). However, we do not know if similar differences exist in the early embryo or placenta. To understand the basis of programmed sex differences, we need to carefully examine the epigenetic landscape of male and female embryos, especially the extraembryonic tissues, to determine the developmental basis of the different growth phenotypes. Doing so will shed light on the black box that exists between a range of male exposures and the sexually dimorphic outcomes we observe.
Working in this area of research and questioning the established dogma is fun, although, at times, it can be challenging. Nevertheless, our work is important and contributes evidence that we hope, one day, redresses the stigma that FASDs are exclusively of maternal origin. As Father's Day approaches, the weather warms up, and we now reclaim our freedom from Covid, I will (consciously) spend more time with my daughter and contemplate the possibility that her physician may, instead of exclusively focusing on her, ask both her and her partner how much they drink.
References
1. Raley S, Bianchi S. Sons, daughters, and family processes: Does gender of children matter? Annu Rev Sociol 32: 401–421, 2006.
2. Bedi Y, Chang RC, Gibbs R, Clement TM, Golding MC. Alterations in sperm-inherited noncoding RNAs associate with late-term fetal growth restriction induced by preconception paternal alcohol use. Reproductive Toxicology 87: 11–20, 2019. doi: 10.1016/j.reprotox.2019.04.006.
3. Chang RC, Skiles WM, Chronister SS, Wang H, Sutton GI, Bedi YS, Snyder M, Long CR, Golding MC. DNA methylation-independent growth restriction and altered developmental programming in a mouse model of preconception male alcohol exposure. Epigenetics 12, 2017. doi: 10.1080/15592294.2017.1363952.
4. Chang RC, Wang H, Bedi Y, Golding MC. Preconception paternal alcohol exposure exerts sex-specific effects on offspring growth and long-term metabolic programming. Epigenetics and Chromatin 12, 2019. doi: 10.1186/s13072-019-0254-0.
5. Chang RC, Thomas KN, Bedi YS, Golding MC. Programmed increases in LXRα induced by paternal alcohol use enhance offspring metabolic adaptation to high-fat diet induced obesity. Molecular Metabolism 30: 161–172, 2019. doi: 10.1016/j.molmet.2019.09.016.
6. Mustapha TA, Chang RC, Garcia-Rhodes D, Pendleton D, Johnson NM, Golding MC. Gestational exposure to particulate air pollution exacerbates the growth phenotypes induced by preconception paternal alcohol use: a multiplex model of exposure. Environ Epigenet 6: dvaa011, 2020. doi: 10.1093/eep/dvaa011.
7. Thomas KN, Zimmel KN, Roach AN, Basel A, Mehta NA, Bedi YS, Golding MC. Maternal background alters the penetrance of growth phenotypes and sex-specific placental adaptation of offspring sired by alcohol-exposed males. FASEB J 35: e22035, 2021. doi: 10.1096/fj.202101131R.
8. Vassoler FM, White SL, Schmidt HD, Sadri-Vakili G, Pierce RC. Epigenetic inheritance of a cocaine-resistance phenotype. Nat Neurosci 16: 42–47, 2013. doi: 10.1038/nn.3280.
9. Schrott R, Murphy SK. Cannabis use and the sperm epigenome: a budding concern? Environ Epigenet 6: dvaa002, 2020. doi: 10.1093/eep/dvaa002.
10. Wimmer ME, Briand LA, Fant B, Guercio LA, Arreola AC, Schmidt HD, Sidoli S, Han Y, Garcia BA, Pierce RC. Paternal cocaine taking elicits epigenetic remodeling and memory deficits in male progeny. Mol Psychiatry 22: 1641–1650, 2017. doi: 10.1038/mp.2017.8.
11. McGrath J, Solter D. Completion of mouse embryogenesis requires both the maternal and paternal genomes. Cell 37: 179–183, 1984.
12. Eriksson JG, Kajantie E, Osmond C, Thornburg K, Barker DJP. Boys live dangerously in the womb. Am J Hum Biol 22: 330–335, 2010. doi: 10.1002/ajhb.20995.
13. Rinn JL, Rozowsky JS, Laurenzi IJ, Petersen PH, Zou K, Zhong W, Gerstein M, Snyder M. Major molecular differences between mammalian sexes are involved in drug metabolism and renal function. Dev Cell 6: 791–800, 2004. doi: 10.1016/j.devcel.2004.05.005.
14. Waxman DJ, Holloway MG. Sex differences in the expression of hepatic drug metabolizing enzymes. Mol Pharmacol 76: 215–228, 2009. doi: 10.1124/mol.109.056705.
Published March 2022

By Shashi N. Kumar, PhD
This blog is also available on our Medium Channel.
Scientists everywhere are adapting to the “new normal” in the age of COVID-19 as the world around us demands rapid answers to complex questions. The current situation has shone a light on the importance of exposure-related research and has revealed the critical need for crosstalk between public health experts, the medical community, laboratory-based research scientists, and the general public. Several of the important questions to arise during this pandemic focused on the dangers of viral infection to pregnant women and their developing offspring. The importance of exposure science in the context of pregnancy and birth defects research has been underscored by the COVID-19 crisis. Whether the exposure is viral, bacterial, psycho-social, nutritional, or environmental, we know that understanding the risks during pregnancy to the mother and developing offspring is paramount to protecting human health. Below is a discussion of my previous and current research as well as my future research interests.
I began my research on pathological changes in combined toxicity of the pesticides endosulfan and ochratoxin- A (OTA) in adult male Wistar rats as a postgraduate (Master of Science) student under the guidance of Prof. Rambir Singh, Bundelkhand University, Jhansi and Dr. Avinash Gopal Telang, Centre for Animal Disease Research and Diagnosis, Indian Veterinary Research Institute, Izatnagar, Uttar Pradesh, India. This study was designed to address the concern that in real life, organisms are concurrently exposed to several i.e., more than one pollutant or toxin. Therefore, from this study, I had drawn the following conclusions that low doses of OTA @ 4 mg/kg BW and endosulfan @ 5 mg/kg BW either alone or combined for 30 days caused significant toxicity in adult rats. The results suggest that both toxins (OTA and endosulfan) had additive effects in rats.
Before becoming a doctoral student, I worked as a research fellow at Environmental Toxicology, ICMR-National Institute of Pathology. During my fellowship, I was inspired by the passion and dedication of each of my co-workers to truly give out to the public and challenge myself to fill in their shoes one day. Dr. S. Sriramachari was the Founder Director of the Indian Registry of Pathology, later named Institute of Pathology (ICMR) and is now called the ICMR- National Institute of Pathology (ICMR), New Delhi. He was the first in India, who used the human placenta as a dual biomarker for monitoring fetal and maternal environments with special reference to potentially toxic environmental pollutants. He headed the committee on Bhopal Gas Tragedy and research on the toxic effects of gas leakage and submitted the report on it to the government of India. So, I decided to pursue a doctorate in Toxicology with the intent to work on research that will support the implementation of sound, scientific policy for public health. I obtained my PhD degree entitled 'Study of Toxic Manifestations of Exposure to Pesticides in Workers in Tea Gardens in North Eastern State of India' in 2021 atICMR-National Institute of Pathology and Jamia Hamdard, New Delhi, India with a dissertation in real dynamics, under the supervision of distinguished Scientist Dr. Arun Kumar Jain and Professor (Dr.) S Raisuddin. Both my supervisors always stood by my side and guided me through all the difficulties while doing my research studies. During my doctoral study as a research scholar, I did a lot! The majority of time was spent designing and conducting the various experiments, analyzing the data, and interpreting results. While pursuing my PhD career in ICMR-National Institute of Pathology, I was also inspired by one of my senior colleagues, Dr. Banajit Bastia who was already working in the same Lab on a thought-provoking problem of "Effect of Tobacco use (Nicotine) on the Fetus (low birth weight babies) due to tobacco consuming mothers with special reference to Placental changes. I used to see him passionately working in the Lab and conducting his experiments relentlessly with great determination. He used to discuss with me whenever he faced the problem with experimental as well as analytical issues and we try to solve it by amicable scientific conversation. This scientific exchange of thoughts and knowledge with him regularly on the wider environmental issues generated curiosity in me and probably imbibed the much needed scientific temperament in me. This inspired me during my early days in the ICMR-National Institute of Pathology to take on this challenging field of research on environmental toxicology.
All this would not have been possible in my life, especially the achievements of my research career, without the help rendered by my Father (Mr. Harpal Singh) and Mother (Mrs. Murti Devi). I owe a lot to them, as this was their dream to see me as a PhD scholar and do some research for the welfare of society.
Detailed studies undertaken in my research career with the outcome until now is provided in a nut-shell as below:
Pesticides are globally used to eliminate pests from crops and plants. The increased use of pesticides has posed a serious threat to human health. This study evaluated the effects of pesticide exposure on pregnancy outcomes in tea garden workers. Tea (Camellia sinensis L. O. Kuntze) is one of the most cultivated
perennial cash crops in Assam, a state in India. More than51% of the tea produced in India is cultivated in Assam, which accounts for around 1/6th of the worldwide production of tea. Generally, the spraying of pesticides is handled by men, whereas the collection of tea leaves is done by women workers. This arrangement directly exposes both men and women to the pesticides used.
My dissertation explored whether female tea garden workers were more vulnerable to pesticides exposure than housewives because female tea garden workers were directly exposed to pesticides during pregnancy. Pesticides such as heptachlor, endosulfan sulfate, phosalone, imidacloprid etc., cross through the placenta, enter the fetal blood and accumulate in the fetus, subsequently causing adverse health effects. In addition, some pesticides accumulate in the placenta and cause potential alterations in the development or functions of placental structures resulting in adverse effects on fetal development. Results suggested that occupational pesticide exposure during pregnancy may decrease acetylcholinesterase (AChE) activity and cause in utero pathological changes accompanied by an increased hypoxia-inducible factor-1a (HIF-1α) expression, which also contributes to placental insufficiency and fetal growth restriction. Taking into consideration the vulnerability of the embryo and the fetus to environmental chemicals, our results are of great concern and need for a follow-up study. If researchers do proper follow-up study then researchers can have ample potential data which can help the government for making policies to prevent the exposure of pesticides in female tea garden workers. Economically tea plantation workers are not very sound, illiterate and socially backwards. Thus the females don't pay attention and can not afford to have proper and nutritious diets during their pregnancy and have to work throughout the pregnancy, which affects placental physiology and fetal development. The finding of the thesis illustrated that female tea garden workers have low socioeconomic status. The information regarding socioeconomic status was already studied and known facts but it become more manifested with my further research. Therefore, the income of female tea garden workers should be increased to lead comfortable and hygienic lifestyles, and invest more in health and education. This study also suggested that there is a need for awareness about the potential hazards of occupational exposure to pesticides and the benefits of protective equipment for tea garden workers.
As a graduate student, I enjoyed my experience because it allowed me to think both critically and creatively, to hone my skills as a scientist, to connect with numerous research interest groups and societies, to delve deeply into topics of interest, and learn from others. Travelling to and presenting at scientific meetings was another part of the graduate student experience that I truly appreciated. Something I learned about myself through presenting at meetings is that I have a real passion for communicating science! Whether it’s in writing or aloud, conveying scientific concepts and findings to a diverse array of audiences is so rewarding, and there are ample opportunities to do so as a graduate student.
I published various research papers in esteemed journals with high impact factors from my PhD work. Also, I had been awarded the best oral presentation award and a young scientist award for my research by the Electron Microscope Society of India and Geno-Pro-Invertis University, Bareilly, India.
More About The Author
I am a research fellow at Environmental Toxicology and Electron Microscopy Laboratory, ICMR- National Institute of Pathology, Safdarjung Hospital Campus, New Delhi, India with an interest in contributing to a deeper understanding of how chemicals cause placental toxicity and to determining new strategies to prevent adverse effects on pregnancies. My research is focused on investigating the use of a combination of animal/cell models and biomarkers to investigate how early life exposures affect the development, function, and subsequent health of the offspring. I favour interdisciplinary approaches to biological questions and have incorporated analytical biochemistry as well as cell biological and biochemical approaches in my research. In the current situation, I am working as a postdoctoral fellow on a project entitled “Developing a predictive model for the early detection of Intrauterine Growth Restriction in new-borns from polycyclic aromatic hydrocarbons (PAHs) concentration in maternal blood”. Based on our previous research (Kumar, et al. 2020), we are trying to estimate the PAHs exposure in mothers during pregnancy and their toxic effects of PAHs on newborn babies. Further, this study will try to develop a predictive model through machine learning for the early detection of intrauterine growth restriction (IURG) in newborns from PAH exposure.
Future Research Interests
Looking ahead, I am very interested in pursuing a career related to environmental health at the intersection of public health, primary research, and policy. The mechanistic causes of a majority of adverse effects on developmental organisms remain unknown but are believed to be multifactorial, having multiple levels of control involving genetic, chemical, dietary and environmental factors. Therefore, the overall goal of my future research interest is to elucidate the mechanisms underlying the developmental toxicity of environmental chemicals and drugs in animal models and humans. Also, my research focus will be on understanding the role of cell signalling pathways (like- P-38, endothelial receptor, Nod-like receptor and inflammasome family members) in placental tissues and in regulating pregnancy outcomes, including those complicated by chemical, dietary, genetic, zika virus, coronavirus and environmental factors.
Although this next step has yet to be determined, I am confident that my involvement in the Society for Birth Defects Research and Prevention (BDRP) will help me get my foot in the door, whether through networking opportunities or professional development. I feel that I have already benefitted from being part of BDRP; Recently my research (Structural changes, increased hypoxia, and oxidative DNA damage in placenta due to maternal smokeless tobacco use; 20 July 2021 https://doi.org/10.1002/bdr2.1941) from my doctoral work has been published in the BDRP journal, Birth Defects Research.
More About The Society For Birth Defects Research And Prevention (BDRP)
To understand and prevent birth defects and disorders of developmental and reproductive origin, BDRP promotes multi-disciplinary research and exchange of ideas; communicates information to health professionals, decision-makers, and the public; and provides education and training.
Scientists interested in or already involved in research related to topics mentioned in this blog are encouraged to join BDRP and attend the being held virtually this year in June. BDRP is the premier source for cutting-edge research and authoritative information related to birth defects and developmentally mediated disorders. Our members include those specializing in cell and molecular biology, developmental biology and toxicology, reproduction and endocrinology, epidemiology, nutritional biochemistry, and genetics, as well as the clinical disciplines of prenatal medicine, paediatrics, obstetrics, neonatology, medical genetics, and teratogen risk counselling. In addition, BDRP publishes the scientific journal, Birth Defects Research. Learn more at http://www.birthdefectsresearch.org. Find BDRP on LinkedIn, Facebook, Twitter and YouTube.
Published January 2022

By Madhumita Basu, PhD, MS, Nationwide Children’s Hospital
This blog is also posted on Medium.com.
“Not every birth defect is associated with something we can do to prevent it. But those we can prevent, we should.” – Dr. Hema DeSilva.
I recently came across a striking statistic reported by Christopher Flavelle in his New York Times article on June 18th, 2020. Reviewing 68 studies which include more than 32 million births in the United States, investigators in this issue of JAMA Netw Open. 2020;3(6): e208243; doi:10.1001/jamanetworkopen.2020.8243 have reported that 48 studies on air pollutants and 9 studies on heat exposure had significant association with adverse birth outcomes. These metadata revealed that environmental perturbations exacerbated by air pollutants have a direct consequence on the health of pregnant women, with such exposures being more likely to result in infants who are premature, underweight, or stillborn. Notably, studies that have examined fine particulate matter (PM2.5) found a consistent positive association with preterm birth and low birth weight. Because pregnant women are at higher risk of altered cardiopulmonary physiology by air pollutants and other environmental teratogens, it is absolutely critical to understand the impacts of climate change on pregnancy outcomes at all gestational ages.
The association between maternal exposure to teratogens and an increased occurrence of structural birth defects has been recognized for almost 60 years now. Some of the seminal works from Josef Warkany, Harold Kalter, James G. Wilson, James J Nora and many other investigators in the early to mid-1960’s have recognized that, apart from genetic risk factors, maternal environment plays a vital role in fetal development and congenital malformations. Even with this long-standing literature and existing public health guidelines, continuing efforts have been made to prevent teratogen-induced birth defects. Presently, we agree that the challenges of studying key molecular events underlying human birth defects are two-fold. First, the ethical concern of human fetal tissue research and second, the pleotropic nature of a teratogen.
A recent news feature article in Nature, September 2021 issue by Kendall Powell has addressed a 14-day rule, first proposed in 1979 as a limit on how long after fertilization human embryos can be grown in culture (Nature. 2021 Sep;597(7874):22-24). This 14-day rule was firmly established in the research community based on the time when the primitive streak (a structure that marks the point at which the embryo sets up the body axes) first appears in the embryo, i.e., shortly before gastrulation, which begins around 14 days in humans. In May 2021, the International Society for Stem Cell Research (ISSCR) released a new set of guidelines that relaxed this 14-day rule, allowing developmental biologists to study gastrulation to understand the mechanisms of cell differentiation into specialized tissues and organs (Lovell-Badge, R. et al. Stem Cell Rep. 16, 1398–1408 (2021)). The relaxation of the 14-day rule empowers researchers to study lab-grown human embryos post-fertilization. These revised guidelines permit researchers to better understand the important stages of human embryo development and enable scientists to learn origins of birth defects and why some pregnancies fail. I would encourage the early career birth defect researchers and developmental toxicologists to be aware of the huge possibilities ahead of us. The breadth of data likely to be obtained from human fetal tissue research after “lifting restrictions” (generated responsibly and thoughtfully), human induced pluripotent stem cell derived cells, and transgenic animal models would certainly facilitate better understanding of disease causation and might mitigate certain birth defects.
The other challenge of birth defects research is tissue or cell-type specific responses to a particular teratogen at different developmental stages. The dose, duration, and timing of fetal exposures to teratogens underlies the spectrum and degree of malformations crucial in determining the complex responses to exposure. Many of the maternal environmental and metabolic perturbations acting in prenatal life continue to have long-term consequences and compromise the quality of an offspring’s life. One such example is an increased risk of childhood cancer in those with congenital heart disease, suggesting a shared developmental origin for the diseases (Birth Defects Res. 2018 Oct 16;110(17):1314-1321). Although I have highlighted one specific example here, the implications of such correlations are huge; further investigation is needed to elucidate the multifactorial etiology of developmental origins of disease. I am a firm believer of basic scientific research and think that, by amalgamating the knowledge of clinical association between teratogenic exposure and birth defects with targeted disease modelling in rodent models (in vivo), in specialized cell lines (in vitro, hiPSCs) and using a systemic approach (in silico), we could better manage adverse pregnancy outcomes and fetal phenotypes. Till date, teratogen-induced birth defects are categorized as; 1) Physical teratogens (ionizing agents, hyperthermia); 2) Metabolic conditions (diabetes, obesity, malnutrition, thyroid disorders, phenylketonuria); 3) Maternal Infections (e.g., rubella virus, herpes simplex virus, Zika virus, and syphilis); and 4) Chemical and drug exposures (e.g., alcohol, tobacco, organic mercury compounds, polychlorinated biphenyls, as well as some pesticides/herbicides, industrial solvents, prescription and over the counter drugs, and illicit and recreational drugs). Due to impacts of the climate crisis discussed earlier and living amidst a global pandemic for last two years, unfortunately this list is rapidly expanding. Studies have reported higher rates of unfavorable birth outcomes in women infected with SARS-CoV-2 in late pregnancy (Am J Obstet Gynecol MFM. 2020; 2:100107). While these association studies are preliminary in nature, there is an urgent need to continue monitoring long-term implications of the clinical cases of COVID-19 infection during the first and early second trimester of pregnancy. This will certainly advance our understanding linking the role of COVID-19 on fetal and postnatal development (J Glob Health. 2020 Dec; 10(2): 020378).
Independent studies across the world have indicated that there are several considerations in determining the type and amount of damage to the developing fetus resulting from exposure to a particular teratogen or combination of more than one teratogen (Berger, 2005). https://ilearn.laccd.edu/courses/133116/pages/harm-to-fetus-teratogens. This warrants more careful study design to address the nature of perturbations in the maternal-fetal interface before we define “exposome (maternal exposures)-phenome (fetal phenotype) association.” The precise mechanism(s) through which the intrauterine environment may impact various cell types in the fetus, during development, is not clear. With rapid advancements in single cell sequencing technologies, bioinformatics analysis, and multi-species data integration, investigators are now capable of cataloging the teratogenic impact on individual cell types; for example, during heart, brain, and limb development. Single cell and spatial multi-omics platforms will serve as important tools for advancing the field of Birth Defects Research. For example, Cao et al. (Science. 2020 Nov 13;370(6518): eaba7721) used a total of 5 million cells to catalogue the single cell atlases of both gene expression and chromatin accessibility in 121 human fetal tissues representing 15 organs. This research serves as a rich resource for the exploration of human gene expression in diverse tissues and cell types. These studies are of utmost significance for understanding fetal development in a “normal maternal environment” and should be considered before embarking on research projects dissecting the mis-regulation of genes following teratogenic exposure. In closing, birth defects do affect society, the healthcare system and, most importantly, families with children who have such defects in many different ways. Therefore, we have social responsibility and need to act now! Global, multicentric, interdisciplinary measures should be undertaken to create human birth defect atlases similar to one mentioned above. Utilizing all the knowledge we accumulate, we should be able to better manage the pediatric patients with teratogen-induced birth defects.
About the Author
Madhumita Basu, PhD, MS is a Principal Investigator at the Center for Cardiovascular Research in Nationwide Children’s Hospital and Research Assistant Professor at the Department of Pediatrics, The Ohio State University College of Medicine. She joined Nationwide Children’s Hospital in 2013. Madhumita’s research is mainly focused on understanding the key molecular mechanism(s) of abnormal embryonic heart development when exposed to pre-existing maternal diabetic environment, which contribute to elevated risk of congenital heart defects.
2021
Published May 2021

By Vinita Oberoi Leedom, MPH, APM, PMP
Member of the Society for Birth Defects Research and Prevention
This blog is also published on Medium.com.
Last Mother’s Day, I had the privilege of bringing home a beautiful baby girl I named Anika. She was, and still is, the brightest spot in the pandemic for me. Whenever my baby girl smiles at me, her dark eyes warm and trusting, it feels like the sun is peeking through the clouds.
My pregnancy with Anika was filled with reminders of the importance of what you do every day, wherever you are, in the busy and quiet corners of the world we live in. As toxicologists, researchers, geneticists, public health workers, policy makers and more, the work you do means the difference between illness and health for current and future generations. I am so grateful for your dedication to your field. I am thankful for the medical advances that have come about because of the persistent curiosity of people like you. From my first prenatal visit when my astute physician surveyed me about folic acid intake, alcohol habits and genetic testing, to immunizations in the second trimester and Group B streptococcus testing at 37 weeks, my care teams were thinking about me and my daughter’s health in ways that only came about because of the work of scientists and data translators like you.
In research, we don’t always get to see the fruit of our labor. Once upon a time, people thought Ignaz Semmelweis was crazy, but he is credited for decreasing the incidence of infection among laboring women. I’m sure some of the other monks thought Gregor Mendel was a little strange for staring at pea plants for hours on end, but he laid the foundation for modern genetics. Rous had to wait fifty years for anyone to credit him for his discovery that viruses can play a role in cancer development. However, without these people, we wouldn’t have the advances we have now. We wouldn’t have the opportunity to seek out treatments for things that were considered completely incurable just one hundred years ago. Without people like them and you, my daughter wouldn't have been screened for life-threatening diseases with a simple heel prick at one day old.
Whether you are drafting a policy statement on vaccination right now or puzzling over the physiology of a zebrafish, please know that you are inspiring, your work matters, and the world needs you. Your work communicates that human beings are valuable, and that we must protect them. This Mother’s Day, I thank you on behalf of new mothers like me for your dedication to cutting edge research that ultimately protects our children and those to come. We have a lot to celebrate and appreciate your continued work in this field.
About the Author
Vinita manages a statewide birth defects surveillance program in SC, USA. She is also a doctoral candidate in public health at USC SC, and is studying receipt of healthcare services among children with special needs. Vinita is mom to Anika (almost one!) and David, 7.
More about the Society for Birth Defects Research and Prevention (BDRP)
To understand and prevent birth defects and disorders of developmental and reproductive origin, BDRP promotes multi-disciplinary research and exchange of ideas; communicates information to health professionals, decision-makers, and the public; and provides education and training.
Scientists interested in or already involved in research related to topics mentioned in this blog are encouraged to join BDRP and/or attend or present their research at the Society’s virtual 61st Annual Meeting this summer. BDRP is the premier source for cutting-edge research and authoritative information related to birth defects and developmentally mediated disorders. Our members include those specializing in cell and molecular biology, developmental biology and toxicology, reproduction and endocrinology, epidemiology, nutritional biochemistry, and genetics, as well as the clinical disciplines of prenatal medicine, pediatrics, obstetrics, neonatology, medical genetics, and teratogen risk counseling. In addition, BDRP publishes the scientific journal, Birth Defects Research. Learn more at http://www.birthdefectsresearch.org. Find BDRP on LinkedIn, Facebook, Twitter and YouTube.
Published March 2021
By Sonja Rasmussen, MD, MS
Past President of the Society for Birth Defects Research and Prevention
This blog is also published on Medium.com.
So much has changed in the past year—who could have predicted that terms like lockdown, herd immunity, and personal protective equipment (PPE) would become part of the general public’s vocabulary?! The same is true regarding what we know about COVID-19 and pregnancy. Looking back to a year ago, in February of 2020, the news was beginning to be dominated by reports of a novel virus that had emerged in Wuhan, China. By February, I had been following the virus for a while. Because of my years working at the Centers for Disease Control and Prevention (CDC) in the pandemic planning unit, I followed STAT news reporter Helen Branswell on Twitter. On December 31, 2019, she tweeted, “Hopefully this is nothing out of the ordinary. But a @ProMED-mail posting about “unexplained pneumonias” in China is giving me #SARS flashbacks” (Figure). The possibility of a new respiratory virus gave me chills. Could this be the “big one”– the nightmare scenario of a novel respiratory virus spreading easily from person to person—that kept us up at night? Of course, it didn’t take long for us to recognize that this WAS the big one that we’d been preparing for. Two days later, Helen tweeted, “Not liking the look of this.” Helen is an incredibly savvy science reporter -- I had been interviewed by her several times in my days at CDC, and she was a knowledgeable (and tough) interviewer. If Helen was worried, I was worried, too.
I was especially concerned about the effects of this new virus on pregnant people and their babies. I had retired from CDC in June of 2018 after 20 years, during which I had worked on multiple infectious disease outbreaks—2009 H1N1 influenza, H7N9 influenza, Middle East Respiratory Syndrome (also caused by a coronavirus), Ebola, and most recently Zika virus. Working on these outbreaks had taught us about the importance of studying the effects on the pregnant people and their babies. During the 2009 H1N1 influenza pandemic, we learned that the virus had severe effects on pregnant people—pregnant people made up about 5% of deaths in the United States from 2009 H1N1 flu, even though only ~1% of the general population is pregnant. And during the Zika virus outbreak, we learned that babies born after a Zika-infected pregnancy are at risk for severe birth defects, because the virus could pass from the mother across the placenta to the fetus and cause damage.
After Helen’s January 2 tweet, things moved rapidly. On January 7 Chinese scientists identified a new coronavirus as the cause of those mysterious pneumonias, on January 9 China shared the genetic sequence of the virus, and on January 21 the first case was confirmed in the United States. On January 27 I reached out to my close friend and colleague Dr. Denise Jamieson about writing a paper on what we might expect during pregnancy based on what we knew about the effects of SARS (severe acute respiratory syndrome) and MERS (Middle East respiratory syndrome), two previous coronaviruses that had caused severe disease. Denise, now chair of the Department of Gynecology and Obstetrics at Emory, and I had worked together for many years on issues related to infections and pregnancy when we were both at CDC. A few days later on January 30, the World Health Organization declared the outbreak a Public Health Emergency of International Concern. When I look back at the first draft of the paper I sent to Denise on February 1, a lot has changed. At that time, the virus and disease were referred to as 2019 Novel Coronavirus (2019-nCoV) and 2019-nCoV infection, respectively; only 6 cases had been diagnosed in the United States; and no cases of infection during pregnancy had been reported. On February 11, the disease got an official name: Covid-19 (“Co” and “Vi” to indicate that it is caused by a coronavirus, “d” to indicate that it causes disease, and 19 to note the year that the infection had emerged). After many updates, I pushed the “submit” button on our paper on February 12, and by the time we completed the revisions 5 days later, the virus had also gotten a name: severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and 2 reports that described 18 pregnancies had been published. We quickly added the new information and a comment to the paper -- given that this was a rapidly evolving situation, we encouraged obstetricians to follow the CDC website to stay updated. The paper was accepted on February 18 and was published online on February 24.
Almost a year later, on February 8, 2021, we published an update on COVID-19 in pregnancy in JAMA to summarize what we know now about COVID-19 during pregnancy. Although early data were unclear as to whether pregnancy was a risk factor for severe disease, later data showed that pregnant people are at increased risk for admission to an intensive care unit (ICU), for requiring invasive ventilation (a machine to help with breathing), and for death, compared to women of reproductive age. COVID-19’s effects on the newborn are less clear. Several, but not all, studies have suggested that infants born after a SARS-CoV-2-infected pregnancy are more likely to be born preterm. Although the virus can be passed from the mother across the placenta to the fetus, that appears to occur rarely, and no evidence has suggested an increased risk for birth defects. Transmission of the virus through breastfeeding also appears to be unlikely. Early on, concerns were raised about transmission from an infected breastfeeding mother to the baby after birth, but it appears that with appropriate precautions (wearing a mask and practicing careful hand and breast hygiene), transmission can be prevented.
The most exciting news in the area of COVID-19 is the availability of safe and effective vaccines. Currently two vaccines, made using messenger RNA (mRNA) technology, are available in the United States (US), and others are expected to be available soon. As is often the case for clinical trials of medications, pregnant people were excluded from the clinical trials for COVID-19 vaccines. However, given what is known about the safety of other vaccines during pregnancy and about how mRNA vaccines work, there is no reason to expect that these vaccines would pose a risk to the fetus. Therefore, several groups, including CDC, American College of Obstetricians and Gynecologists (ACOG), and the Society for Maternal-Fetal Medicine (SMFM) have come forward to say that pregnant persons who meet criteria for receiving COVID-19 vaccine may choose to receive the vaccine. Pregnant persons might benefit from a discussion with their physician to weigh the benefits and potential risks of the vaccine during pregnancy, although this discussion should not be required before vaccination. Several studies are in progress to better understand the effects of COVID-19 vaccines during pregnancy, so more information is expected soon. Data on the effects of COVID-19 vaccines on the breastfed baby are also not available. However, given what is known about the safety of breastfeeding after other types of vaccines and the well-recognized benefits of breastfeeding to the infant and mother, CDC, ACOG, and SMFM are all reassuring about receiving the vaccine while breastfeeding.
It’s been a whirlwind year, filled with uncertainty for all including pregnant people. Much has been learned about COVID-19 and pregnancy over the past year, but many questions remain. For example, beyond prematurity, are there other effects on a baby born after a SARS-CoV-2 infected pregnancy? And more data are needed on COVID-19 vaccines during pregnancy—for example, does giving a COVID-19 vaccine during pregnancy provide protection to the newborn, as has been seen with influenza and Tdap vaccines? I encourage researchers to continue to challenge themselves to answer these critical questions and applaud those clinicians who translate the currently available data to their patients. By working together, I am hopeful that many of these questions will be answered soon.
About the Author
 Sonja A. Rasmussen, MD, MS, is professor of pediatrics, obstetrics and gynecology, and epidemiology at the University of Florida College of Medicine and College of Public Health and Health Professions. She joined the University of Florida after 20 years at the Centers for Disease Control and Prevention (CDC), where she worked on public health responses to 2009 H1N1 influenza, Middle East Respiratory Syndrome (MERS), Ebola, and Zika viruses.
Sonja A. Rasmussen, MD, MS, is professor of pediatrics, obstetrics and gynecology, and epidemiology at the University of Florida College of Medicine and College of Public Health and Health Professions. She joined the University of Florida after 20 years at the Centers for Disease Control and Prevention (CDC), where she worked on public health responses to 2009 H1N1 influenza, Middle East Respiratory Syndrome (MERS), Ebola, and Zika viruses.
More about the Society for Birth Defects Research and Prevention (BDRP)
To understand and prevent birth defects and disorders of developmental and reproductive origin, BDRP promotes multi-disciplinary research and exchange of ideas; communicates information to health professionals, decision-makers, and the public; and provides education and training.
Scientists interested in or already involved in research related to topics mentioned in this blog are encouraged to join BDRP and/or attend or present their research at the Society’s virtual 61st Annual Meeting this summer (abstracts due March 31). BDRP is the premier source for cutting-edge research and authoritative information related to birth defects and developmentally mediated disorders. Our members include those specializing in cell and molecular biology, developmental biology and toxicology, reproduction and endocrinology, epidemiology, nutritional biochemistry, and genetics, as well as the clinical disciplines of prenatal medicine, pediatrics, obstetrics, neonatology, medical genetics, and teratogen risk counseling. In addition, BDRP publishes the scientific journal, Birth Defects Research. Learn more at http://www.birthdefectsresearch.org. Find BDRP on LinkedIn, Facebook, Twitter and YouTube.
Published February 2021

By Elise M. Lewis, PhD
This blog is also published on Medium.com.
Recently I was interviewed by a local news network that was working on a Black History series celebrating living history. This was indeed a surprise and an honor, and I must extend my gratitude to Dr. Maia Green for recommending me for the momentous opportunity. Prior to this interview, if I were asked what Black History Month meant to me, I’d simply reply “it is a time to reflect on my culture and family traditions, remember the legacy of those who have left an indelible mark on this nation, and celebrate with pride the many contributions of African Americans in the areas of science, education, sports, politics, and social justice, to name a few.” That remains true, but today, I would proclaim that it’s high time that we celebrate the successes of others while we have the opportunity. Tomorrow is too late, and a month is not long enough.
In the days leading up to the interview, the thought bubbles were running rampant in my mind. “What questions will I be asked?”, “Do I need to prepare notes?,” “What should I wear?,” “What about my hair!!!?” Considering that I have been donning an athleisurely look throughout the pandemic, I did spend some quality time reacquainting myself with my closet and envisioning what impression I wanted to make. After I realized that the span of the interview would be condensed to a 2- to 4-minute highlight, I took a deep breath to move past the initial excitement and compartmentalized the moment (to the best of my ability) until the day of the filming. The purpose of this interview was to celebrate black women in science who should be remembered in the future. I approached it as an opportunity to tell my story to a broader audience and to deliver a message that would inspire hope for future generations of scientists. Although I didn’t write out a script (hard to do for an interview), I did jot down a few notes and key phrases so that my message would be clear and impactful. Fast forward to the day of the interview, lights, camera, mic check, and action.
Growing up in the south as a child of a tireless educator I was frequently reminded of my family history, our cultural diversity, and the importance of education and how it could shape my future. If my mother were here today to see how her strength, love, leadership, insightfulness, and drive for academic excellence would shape my womanhood and the scientist that I have become she would be speechless to say the least, but quickly remind me that I have many miles to go on my journey.
From conception, my life has been a unique journey, and my career as a scientist continues to evolve. I am making history, not just black history and not solely for myself, but for the multitude of underrepresented minorities who need a role model to empower and encourage them to pursue education and a career in STEM. Throughout my journey I have been labeled the ‘first’ and often ‘the only’ in many facets of my life. Early in my academics, I was one of few in advanced placement classes offered in high school and at the time I graduated from the University of Alabama I was one of two African American females who was bestowed the honor of a doctorate degree in biological sciences. As an aside, I was second only to my best friend whose last name came before mine in the alphabet. In my family, where educate is of utmost importance (as evidenced by the plaques we receive for academic achievements), I was the first to graduate with a doctorate degree and the third grandchild to graduate from college. In my career, I can count the number of African Americans who focus their scientific efforts on Reproductive, Developmental, and Juvenile Toxicology on my hands. And most notably, I am the first African American elected and to serve in the presidential line of our Society for Birth Defects Research and Prevention and of the Philadelphia Chapter of the Association for Women in Science. While I could go on and on, I do not let labels such as ‘first’, ‘only’, and ‘one of few’ detour me from living a life full of purpose personally or professionally. The uniqueness in my daily walk allows me to use my resources, networks, and platforms to promote diversity, equity, and inclusiveness within my discipline and the broader STEM community, be a catalyst for change, empower the scientific leaders of tomorrow, and to pay my success forward through mentorship and sponsorship.
Research suggests that most underrepresented minorities attribute their absence or departure from STEM occupations to lack of access to quality education, lack of role models, uncertainty of success, discrimination in recruitment practices and promotions (whether conscious or unconscious), and lack of encouragement to pursue STEM from an early age. Such barriers, whether real or not, can be prohibitive, do not foster diversity or inclusion, and limits one’s ability to take steps toward realizing their full potential. I have met many underrepresented minority students/trainees with knowledge, gifts, and talents who are in need of an opportunity to help them flourish and to visualize a better future in STEM. Through mentorship, advocacy, and community service, I chose to be a guiding light and a voice of what is possible rather than a mere echo.
Mentors are invaluable assets who can equip, motivate, inform, inspire, and believe in us as we navigate a course to our destiny. The path I traveled to achieving my dreams was not necessarily a straight line. The detours I encountered, while momentary, helped me to develop the strength, character, and skills I needed to navigate successfully through my journey. But success is rarely achieved alone. I’ve certainly had many mentors, and I am forever grateful to the mentors of my past and my present who guided me, built my confidence, opened a door, and gave me the momentum necessary to move forward. They were, and many still are, true success partners who inspire me to reach beyond what I can see.
And cut! Now, queue up the snow storm. While I wasn’t able to share my entire life’s story during the interview, I did hone in on my field of research, what inspired me to enter the field of science, my greatest achievement to date, and the power and rewards of good mentorship. If I could rewind a single moment in the interview I would redo my answer to “Do you have any final words to say to young African American boys or girls who may be inspired by your story?” At the time I was not prepared for this question because I have miles to go before I complete my scientific journey. Now that I have had time to think more about the question my response is simply “The Best Is Yet To Come.”
Each of us has a story worth sharing about our personal pursuit of education and a career. Your passion for science could be the catalyst that ignites someone’s future in STEM. Are you ready to tell your story?
More about the Society for Birth Defects Research and Prevention (BDRP)
To understand and prevent birth defects and disorders of developmental and reproductive origin, BDRP promotes multi-disciplinary research and exchange of ideas; communicates information to health professionals, decision-makers, and the public; and provides education and training.Scientists interested in or already involved in research related to topics mentioned in this blog are encouraged to join BDRP and/or attend or present their research at the Society’s virtual 61st Annual Meeting this summer (abstracts due March 31). BDRP is the premier source for cutting-edge research and authoritative information related to birth defects and developmentally mediated disorders. Our members include those specializing in cell and molecular biology, developmental biology and toxicology, reproduction and endocrinology, epidemiology, nutritional biochemistry, and genetics, as well as the clinical disciplines of prenatal medicine, pediatrics, obstetrics, neonatology, medical genetics, and teratogen risk counseling. In addition, BDRP publishes the scientific journal, Birth Defects Research. Learn more at http://www.birthdefectsresearch.org. Find BDRP on LinkedIn, Facebook, Twitter and YouTube.
Published January 2021

By Lauren Walker, PhD
This blog is also published on Medium.com.
From anti-anxiety gummies to infused “wellness water,” cannabidiol (CBD) popularity has skyrocketed in the personal care, wellness, and food industries. CBD is one of the main cannabinoids found in cannabis plants such as marijuana and hemp. Starting in the late 1990s, paradigm shifts in public viewpoints on cannabis drove the gradual legalization of marijuana for medical use and spurred subsequent interest in the potential for cannabis-derived therapeutics. In my wellness-orientated home state of California, CBD products are ubiquitous in personal care aisle and health stores. Product users often tout CBD as a “wonder supplement” for a variety of ailments—including pregnancy discomforts.
Positive anecdotes are on the rise in personal blogs, mainstream publications, and product review pages where users claim reduced anxiety, improved sleep, and eased labor pain. While not all of CBD's purported health claims have been substantiated, some research suggests that CBD may effectively suppress two common pregnancy symptoms: nausea and vomiting. Naturally, growing numbers of pregnant women have tried or considered trying CBD products to relieve pregnancy symptoms. A recent survey found that 20% of women would consider using any form of CBD during pregnancy and 29% of women believed topical CBD was safe. However, the soaring popularity of CBD products is alarming. We currently lack clear and sufficient information regarding the risks posed, if any, by CBD use during pregnancy.
What is CBD and where is it found?
Cannabis is characterized by a unique class of chemicals known as cannabinoids. CBD is the second-most prevalent cannabinoid found in cannabis after the psychoactive THC (delta-9-tetrahydrocannabinol). While CBD is often associated with marijuana, it is usually extracted from low-THC hemp cannabis. CBD is featured in a myriad of products including tinctures, "supplement" capsules and gummies, concentrates, topicals (e.g., lotions, salves, lip balms, cosmetics), sprays, vape products, and food stuffs (e.g., soda, candy, water).With the exception of Epidiolex, a prescription drug to treat rare severe seizure disorders in children, CBD is not approved for use as a drug product by the U.S. Food and Drug Administration (FDA). Labels on wellness products, however, may be misleading with regard to promised benefits to consumers. In November 2019, the FDA sent 15 companies warning letters for selling CBD-containing products in violation of the Federal Food, Drug, and Cosmetic Act (FD&C Act). While an early review by the World Health Organization (WHO) in 2018 determined both oral and topical CBD oil use as generally safe under certain circumstances, the FDA has also expressed concerns about the lack of data supporting the general safety of broader CBD use in human and animal food products.
What do we know about CBD use during pregnancy?
Previous safety studies, focused extensively on cannabis use during pregnancy, found that smoking cannabis during pregnancy is linked to lower birth weight, which may cause serious health problems for some babies. Comprehensive safety data and research on CBD specifically is limited, though early findings suggest CBD as a potential hazard to healthy pregnancies.
Early clinical investigations indicate that general CBD use may pose a risk of liver damage or adverse drug interactions in users. That latter is particularly concerning for women who receive anesthesia during labor. In animals, CBD was associated with reproductive toxicity in male offspring of animals exposed during pregnancy. Past BDRP President Dr. Lewis Holmes is currently leading work studying the impact of the CBD-based pharmaceutical Epidiolex on pregnancy outcomes.
Personal care or edible CBD products can also be contaminated with pesticides, heavy metals, or toxic mold or have inaccurate CBD content. There are no current requirements to verify CBD purity in products, which leaves the consumer to trust that the manufacturer has done their due diligence in producing a truthful and high-quality product.
The relationship between cannabis and CBD use and early childhood development outcomes remains unclear. Due to inconsistencies in CBD product manufacture and knowledge gaps surrounding CBD safety, the FDA generally advises against use during pregnancy.
Is CBD federally or locally regulated?
The last 20 years saw a significant paradigm shift regarding legal and public viewpoints on general cannabis use as well as the use of cannabis-derived products. Between the late 1990s and 2010s, cannabis use became more mainstream and publicly viewed as relatively harmless. Though marijuana cannabis remains on the Drug Enforcement Administration (DEA) Controlled Substances List, its non-psychoactive cousin, hemp—a legal CBD source, was removed in 2018 with The Agriculture Improvement Act.
Summary
While there is some promising evidence regarding medical CBD applications (the first prescription pharmaceutical containing CBD (Epidiolex) was approved by FDA in 2018), more studies are necessary to substantiate purported CBD health claims. Early research provides evidence that CBD use during pregnancy may present more harm than help for maintaining healthy pregnancies, though more research is required to understand the full scope of risk. Until the knowledge gap is closed, the best way to minimize risk is for patients to consult with their medical health provider.
About the Author

Lauren Walker is a Postdoctoral Fellow studying developmental toxicology at the Rutgers University.
More about the Society for Birth Defects Research and Prevention (BDRP)
To understand and prevent birth defects and disorders of developmental and reproductive origin, BDRP promotes multi-disciplinary research and exchange of ideas; communicates information to health professionals, decision-makers, and the public; and provides education and training. Scientists interested in or already involved in research related to topics mentioned in this blog are encouraged to join BDRP. BDRP is the premier source for cutting-edge research and authoritative information related to birth defects and developmentally mediated disorders. Our members include those specializing in cell and molecular biology, developmental biology and toxicology, reproduction and endocrinology, epidemiology, nutritional biochemistry, and genetics, as well as the clinical disciplines of prenatal medicine, pediatrics, obstetrics, neonatology, medical genetics, and teratogen risk counseling. In addition, BDRP publishes the scientific journal, Birth Defects Research. Learn more at http://www.birthdefectsresearch.org. Find BDRP on LinkedIn, Facebook, Twitter and YouTube.
2020
Published August 2020

By Alfred N. Romeo, RN, PhD MotherToBaby, Utah
Society for Birth Defects Research and Prevention (BDRP) Communications Coordination Committee Member
This blog is also available on Medium.com.
As with many jobs, the day in the life of a teratogen information specialist is always interesting. I work with MotherToBaby Utah at the Utah Department of Health in our Title V Maternal and Child Health Block Grant agency. This means that our sister programs, such as Oral Health, the Office of Home Visiting, Utah Birth Defect Network, and others, are also working to improve the health of women and children. Many MotherToBaby affiliates in other states are located within universities, so we are a little different. But we do have a partnership with the University of Utah Department of Pediatrics where our Medical Director, Dr. John Carey (another BDRP member)is housed. OTIS is the professional society that provides the MotherToBaby service via affiliates across North America. To round out our staff, Lynn Martinez (past OTIS President) and Luisa Hansen work part-time on our MotherToBaby Utah team.
I’ve been with MotherToBaby UT for about 11 years now, but with the Utah Department of Health for almost 18 years. I got my start with MotherToBaby Utah when one of my part-time grants, working with young adults with special health care needs, ended and I needed more work to do. Before I was a nurse in public health, I worked in neonatal intensive care units caring for infants born prematurely or with birth defects. As I’ve worked with different populations, infants, young children, teens, and pregnant women. I’ve found a place where I can help two generations at the same time, keeping mom healthy and giving baby the best start possible.
About four days a week I answer telephone calls, texts, chat, and email from our Utah clients and answer their questions about a variety of exposures. Our service receives nearly 7,000 inquiries/contacts a year and we might have twenty to thirty contacts a day.
When we are not answering client questions, we keep busy with professional development, meetings, grant reports, and other activities. My degrees in literature, nursing, and health promotion help with the various roles and grant writing. I schedule and run the MotherToBaby Seminars twice a month, on the second and fourth Wednesdays at 12:30 pm Mountain Time. I’m scheduling speakers, setting up equipment, preparing my own presentations a few times a year, and putting recordings on our playlist. The articles that I like to review during the seminars might be related to a hot topic like air pollution; an exposure for a MotherToBaby fact sheet that I’m developing or editing like phenylephrine; an exposure for a manuscript that I’m working on like propylthiouracil; or an exposure for a MotherToBaby blog or podcast like diabetes. All parts of the research cycle are important for our clients, from finding funding to do the studies, developing studies with good methods to answer gaps in the body of knowledge, recruiting participants, conducting the studies, developing the manuscripts, and finally translating the results for our clients. Our seminars and professional development help us improve our skills and propose research priorities to address the gaps in knowledge.
Technology is helping how we work to improve the health of women and their children. When teratogen information services first started in the 1980s, there were many trips to the library to answer client questions. Today, we have a custom database where we enter our contacts and look up summaries for exposures. It was fun working with the developer to build it and today I am responsible for maintaining it. With other databases such as LactMed®, Reprotox®, TERIS®, and Medications and Mothers’ Milk®, we can quickly find the answer to most questions during a conversation with a mother or provider. A few times a month, we have to search other databases, such as PubMed®, for original studies to piece together the best answer possible, in consultation with our colleagues.
Similar to making sure our database is operating, I’m responsible for making sure that our contracts are in place. We have funding contracts with OTIS, the University of Utah, and Utah Medicaid. We have purchasing contracts for our text tool, chat tool, and phone system. We have data contracts with OTIS and the companies that run our text and chat tools. Dealing with the business side of our service isn’t the most fun, but we have to pay the bills.
Speaking of paying the bills, MTB UT has been spending quite a bit of time on fundraising over the past two years. We lost two teratogen information specialist full-time employees to budget cuts over the past few years and a third in July, leaving us with one full-time and two part time staff. We are busier than ever, regrettably keeping women on hold on the phone more often. We have been writing grant applications, meeting with partners, and looking for new funding opportunities so that we can continue to operate and serve the women and providers in Utah that have relied upon us to answer their questions for more than 35 years. I wanted to remind my OTIS and BDRP colleagues to continue to donate to your professional societies. I’ve taken the opportunity to donate regularly to OTIS through AmazonSmile when I make online purchases. I have also donated directly to OTIS some years. Finally, I’ve set up my trust to donate 1% of my estate to OTIS upon my death. I don’t like to see our affiliate services close so I’ve decided to do something about it. I hope you will consider donation options that work for you.
Like everyone, I have plenty of meetings beyond those with the boss and our team. I’ve been an OTIS member since 2009 and a BDRP member since 2014. I serve as the OTIS Research Committee Co-Chair so there are meetings to plan and projects to complete. I serve as one of the OTIS representatives on the BDRP Communications Coordination Committee. I also serve on the OTIS Meeting Planning Committee and help represent OTIS on the BDRP Program Committee where the work for the annual meeting continues all year long. I join other OTIS members in conference calls to support collaboration with the Association of Maternal and Child Health Programs (AMCHP) and the National Birth Defect Prevention Network (NBDPN). I help look for grant opportunities and help write grants when they are available, such as our recent collaborative effort with the Developmental Neurotoxicology Society (DNTS) for our conference grant from the National Institutes of Health.
My job gives me the regular opportunity to teach on a larger scale, something that I enjoy, beyond the one-on-one interactions with clients. A few times a year I provide lectures with pharmacy students, genetic counseling students, and medical residents. On an even larger scale, we work with our Medicaid agency and the outreach group for Medicaid for pregnant women, called Baby Your Baby in Utah. On the third Friday of the month at noon, I represent Baby Your Baby in a television news interview for a station which is broadcast across Utah. The news anchors know me as Nurse Al who wears the purple scrub top. It is a bit of work to pick a monthly topic, prepare my talking points, get edits from the team, and practice those talking points, never knowing what questions I might get on live television. I’ve been doing those regular interviews for few years. I still get nervous but it is fun. Those interviews can be found at https://kutv.com/features/health/baby-your-baby.
In addition to my full-time job, I have a part-time job, a few hours a month, working for the University of Utah on the Medical Home Portal website. I write and edit content and assist with the resources. The Medical Home Portal is designed for primary care providers, and their families, who care for children with special health care needs. Information is provided to help pediatricians screen for conditions and then manage those conditions that they may not see frequently, from arginase deficiency to fetal alcohol spectrum disorders to traumatic brain injury.
I’m cautiously looking forward to the next few years. I’m hoping we can find funding for our local program so I can keep doing the work that I love. I’m also looking forward to serving OTIS as the President Elect in January 2021. I’ll follow other recent OTIS Presidents who were also members of BDRP, such as Robert Felix, Kenneth Jones, Stephen Braddock, Christina Chambers, and Sarah Običan. I plan to continue to foster our joint work with BDRP and other organizations as we strengthen OTIS as an international organization.
There are many ways to get involved in researching and preventing birth defects and other adverse outcomes for mothers and their children. Some of our colleagues are doing research with animals to look for early warnings of possible birth defects; studying the placenta to determine how it responds to medications and various maternal conditions; watching for patterns of birth defects from pregnancy registries or birth defect registries; conducting clinical trials for various medications; and reviewing the studies to interpret those results for teratogen information specialists, public health professionals, and clinicians. Professionals from various disciplines are encouraged to become involved in research and in their professional societies to improve the health of women and children today and for future generations.
 Alfred N. Romeo, RN, PhD works at the Pregnancy Risk Line, MotherToBaby Utah, a partnership between the Utah Department of Health and the University of Utah. Visit the website at https://mothertobaby.utah.gov/ . The MotherToBaby Utah is an affiliate of the Organization of Teratology Information Specialists (OTIS) / MotherToBaby, a non-profit with affiliates across North America. Utah women with questions or concerns about pregnancy or breastfeeding exposures can call 1-800-822-2229. Outside Utah, please call OTIS at 1-866-626-6847. OTIS is conducting several studies, including studies for COVID-19. Women with or without study exposures can call OTIS to volunteer to participate or learn more by visiting www.OTISpregnancy.org.
Alfred N. Romeo, RN, PhD works at the Pregnancy Risk Line, MotherToBaby Utah, a partnership between the Utah Department of Health and the University of Utah. Visit the website at https://mothertobaby.utah.gov/ . The MotherToBaby Utah is an affiliate of the Organization of Teratology Information Specialists (OTIS) / MotherToBaby, a non-profit with affiliates across North America. Utah women with questions or concerns about pregnancy or breastfeeding exposures can call 1-800-822-2229. Outside Utah, please call OTIS at 1-866-626-6847. OTIS is conducting several studies, including studies for COVID-19. Women with or without study exposures can call OTIS to volunteer to participate or learn more by visiting www.OTISpregnancy.org.
More about the Society for Birth Defects Research and Prevention (BDRP)
To understand and prevent birth defects and disorders of developmental and reproductive origin, BDRP promotes multi-disciplinary research and exchange of ideas; communicates information to health professionals, decision-makers, and the public; and provides education and training.Scientists interested in or already involved in research related to topics mentioned in this blog are encouraged to join BDRP. BDRP is the premier source for cutting-edge research and authoritative information related to birth defects and developmentally mediated disorders. Our members include those specializing in cell and molecular biology, developmental biology and toxicology, reproduction and endocrinology, epidemiology, nutritional biochemistry, and genetics, as well as the clinical disciplines of prenatal medicine, pediatrics, obstetrics, neonatology, medical genetics, and teratogen risk counseling. In addition, BDRP publishes the scientific journal, Birth Defects Research. Learn more at http://www.birthdefectsresearch.org. Find BDRP on LinkedIn, Facebook, Twitter and YouTube.
Published June 2020

By Lauren M. Walker, PhD
This blog is also available Medium.com.
When we examine the role of scientists in their communities historically, we tend to think of them as "neutral" or "impartial" entities. Part of the way of the world, yet somewhat separated from it. Until recently, we researchers—in the spirit of advancing our common goal and fostering a collegial atmosphere—have leaned towards "keeping politics out of" our research labs, conferences, and colleague-to-colleague interactions.
While it is true that we should not allow politics to interfere with conducting robust, ethical research, I doubt we can eschew "politics" entirely. With the outbreak of protests across America in the wake of the deaths of George Floyd, Breonna Taylor, Ahmaud Arbery, and countless others, some individuals have expressed a desire to "escape politics" or claim they are not "trying to be political."
The word "politics" is frequently thrown out with a degree of disdain--but what does it actually mean? One definition: “the study of the ways in which a country is governed.” By this definition, “trying not to be political” is a willingness to opt out of engaging in how your community is shaped. As follows, now more than ever is our time to lean in and embrace every single member of our research community and beyond. If we are to be truly committed to building a strong, inclusive future for science and our country, we need to stand with our fellow humans now and moving forward.
Now is also a time for self-reflection and self-awareness. We need to check our biases, educate ourselves, and—most importantly—engage. Silence speaks volumes—and I'm not strictly referring to social media activity. Check in with your colleagues—how are they feeling? Many people of color are not ok right now. Yet, many people also feel constrained to uphold a calm exterior in order to maintain their professional reputations.
Over the last week, I’ve been actively referring my friends and colleagues to the great wealth of online allyship resources that teach how to build inclusive relationships with members of marginalized communities.
If you’re unsure where to begin, I recommend the following resources as a starting point:
- Guide to Allyship: An introduction to becoming an effective ally.
- Justice in June: A curated guide to becoming an informed ally.
- Resource Roundup: Your Kids Aren't Too Young to Talk About Race
This is by no means an exhaustive list. There are many more resources to help unpack bias and stimulate proactive inclusion efforts. I recommend contacting your university’s office of diversity, equity, and inclusion and local cross-cultural, LGBTQ+, and women’s centers.
If we do not engage, we cannot make change. Scientists are a tough bunch—together we're even stronger.
About the Author

Lauren Walker is a Postdoctoral Fellow studying developmental toxicology at the Rutgers University.
More about the Society for Birth Defects Research and Prevention (BDRP)
To understand and prevent birth defects and disorders of developmental and reproductive origin, BDRP promotes multi-disciplinary research and exchange of ideas; communicates information to health professionals, decision-makers, and the public; and provides education and training.
Scientists interested in or already involved in research related to topics mentioned in this blog are encouraged to join BDRP and attend the Annual Meeting being held virtually this year in June and July. BDRP is the premier source for cutting-edge research and authoritative information related to birth defects and developmentally mediated disorders. Our members include those specializing in cell and molecular biology, developmental biology and toxicology, reproduction and endocrinology, epidemiology, nutritional biochemistry, and genetics, as well as the clinical disciplines of prenatal medicine, pediatrics, obstetrics, neonatology, medical genetics, and teratogen risk counseling. In addition, BDRP publishes the scientific journal, Birth Defects Research. Learn more at http://www.birthdefectsresearch.org. Find BDRP on LinkedIn, Facebook, Twitter and YouTube.
Published May 2020

By Bevin Blake, PhD
Society for Birth Defects Research and Prevention Member Since 2018
This blog is also available on Medium.com.
Scientists everywhere are adapting to the “new normal” in the age of COVID-19 as the world around us demands rapid answers to complex questions. The current situation has shone light on the importance of exposure-related research and has revealed the critical need for crosstalk between public health experts, the medical community, laboratory-based research scientists, and the general public. Several of the important questions to arise during this pandemic focused on the dangers of viral infection to pregnant women and their developing offspring. The importance of exposure science in the context of pregnancy and birth defects research has been underscored by the COVID-19 crisis. Whether the exposure is viral, bacterial, psycho-social, nutritional, or environmental, we know that understanding the risks during pregnancy to the mother and developing offspring is paramount to protecting human health.
My research aimed to use translational tools (e.g. a combination of different scientific approaches) to improve our understanding of the human health effects due to environmental exposures. I am particularly interested in the relationship between early life exposures and chronic adult diseases. I recently earned my PhD in Toxicology and Environmental Medicine from the University of North Carolina at Chapel Hill. I completed my dissertation research as an Intramural Research Training Award (IRTA) predoctoral fellow at the National Institute of Environmental Health Science (NIEHS) in the National Toxicology Program (NTP). During my time as a graduate student and trainee, I did a lot! The majority of my time was spent designing and conducting experiments, analyzing the data, and interpreting the results.
As a graduate student, a typical day for me was generally divided between doing work at the bench (e.g. tissue culture experiments or prepping samples for subsequent biomolecular or chemical analyses), data analytics (e.g. importing data into R, writing code, and performing statistical analyses), science writing (e.g. working on manuscripts or science communication pieces), and meetings (e.g. with my PhD advisor to discuss progress or troubleshooting, my lab to share our data, or other research groups with which I am involved). Towards the end of my PhD, there was far less lab work and far more writing and other computer-based work, but at earlier stages of the journey the scales were tipped heavily in favor of lab work. While I enjoyed the challenges and hands-on nature of wet lab work, I discovered that I really love the challenge of working through complex datasets and analyzing/interpreting the findings.
My interest in toxicology was first piqued during the fall semester of my junior year in college at the University of Mount Union, a small liberal arts college in Ohio. I was dual-majoring in biology and psychology with a general plan to go to graduate school and study something at the intersection of the two, like behavioral neuroscience. My track teammate/roommate at the time was an environmental science major and mentioned she planned to take an elective course called Ecotoxicology. I still needed another biology elective, so I thought it might be fun to take the class with her. I was completely blindsided by my fascination with the relationship between the health of organisms and the environment around them.
When I applied to graduate school for my master’s degree program, I joined Dr. Krista McCoy’s lab at East Carolina University in 2013, where I had the chance to design a very cool project that looked at behavioral programming of rat offspring prenatally over-exposed to endogenous hormones. This proof-of-concept study provided a foundation upon which to build subsequent in utero neuroendocrine disruption studies, and it was right up my alley since it combined aspects of both psychology and biology. As I learned more about the human health consequences of early life exposures, I became interested in the Barker hypothesis. I then applied for PhD programs in toxicology and began my doctoral training at the University of North Carolina at Chapel Hill in 2015. In 2016, I joined the lab of Dr. Suzanne Fenton, a long-time BDRP member who shares a passion for protecting human health through understanding the consequences of developmental exposures.
I enjoyed my experience as a graduate student because it allowed me to think both critically and creatively, to hone my skills as a scientist, to connect with numerous research interest groups and societies, to delve deeply into topics of interest, and learn from others. Traveling to and presenting at scientific meetings was another part of the graduate student experience that I truly appreciated. Something I learned about myself through presenting at meetings is that I have a real passion for communicating science! Whether it’s in writing or aloud, conveying scientific concepts and findings to a diverse array of audiences is so rewarding, and there are ample opportunities to do so as a graduate student.
As a graduate student, I uniquely benefitted from both academic and government trainee settings—UNC Chapel Hill provided me with top-notch coursework and a rigorous toxicology doctoral program while the NTP and NIEHS provided me with access to state-of-the-art core facilities and countless collaborations with experts in diverse fields to support my research. I truly feel immensely privileged to have benefitted from such incredible resources.
Looking ahead, I am very interested in pursuing a career related to environmental health at the intersection of public health, primary research, and policy. Although this next step has yet to be determined [insert shameless self-plug about being on the job market here], I am confident that my involvement in the Society for Birth Defects Research and Prevention (BDRP) will help me get my foot in the door, whether through networking opportunities or professional development. I feel that I have already benefitted from being part of BDRP; I have been granted travel awards to present my doctoral research at the 2018 and 2019 annual meetings and have participated in the Awards Committee and the Communication Coordination Committee, which have been excellent experiences as a trainee!
If you want to attend graduate school (either a master’s degree program or a doctoral program) and do birth defects research, my advice would be to take the initiative with contacting programs, labs, and individual researchers of interest to you. Some might not respond, but establishing an early line of communication will help with name recognition when the graduate school programs are inundated with piles of applications. If the person, school, or lab is local, see if you can schedule a casual meet-and-greet (via Zoom or another video chat platform until in-person meetings are safe)! Anything that you can do to set yourself apart and make a lasting positive impression will go a lot further than lines on a resume. That being said, make sure your application materials have undergone multiple rounds of editing and refining by people other than yourself—it’s a painful and iterative process, but inevitably improves the end product.
Between my master’s and PhD work, I was in graduate school for 7 consecutive years, which was a LOT. While there have been plenty of ups and downs, this was the right path for me. Graduate school can be extremely intimidating, but a strong support network (both professional and personal) and an unrelenting passion for research and discovery are key to sustaining motivation and perseverance!
While the ongoing pandemic has impacted the lives of many, one silver lining is that it has highlighted the importance of research focused on the health and safety of pregnant women and their babies. I’m proud to be a member of this research community, and thankful for the ways in which BDRP has supported our community of medical professionals, public health experts, and scientists!
More about the Author

Bevin Blake recently earned her PhD in Toxicology and Environmental Medicine at the University of North Carolina at Chapel Hill, conducting her research with Dr. Suzanne Fenton at the National Toxicology Program. Bevin’s doctoral research focused on the health effects caused by exposure to per- and polyfluoroalkyl substances (PFAS), using translational science to focus specifically on in utero exposure and the placenta as a target. Bevin is passionate about her research and has won 16 different awards during her time as a PhD student, including the UNC Impact Award, which recognizes graduate student research directly contributing to the educational, economic, physical, social or cultural well-being of North Carolina citizens. In her free time, Bevin is a proud dog mom who loves to spend time outdoors and competes for a local elite triathlon team. Follow her research and professional endeavors on Twitter: @bevthescientist
More about the Society for Birth Defects Research and Prevention (BDRP)
To understand and prevent birth defects and disorders of developmental and reproductive origin, BDRP promotes multi-disciplinary research and exchange of ideas; communicates information to health professionals, decision-makers, and the public; and provides education and training.
Scientists interested in or already involved in research related to topics mentioned in this blog are encouraged to join BDRP and attend the being held virtually this year in June. BDRP is the premier source for cutting-edge research and authoritative information related to birth defects and developmentally mediated disorders. Our members include those specializing in cell and molecular biology, developmental biology and toxicology, reproduction and endocrinology, epidemiology, nutritional biochemistry, and genetics, as well as the clinical disciplines of prenatal medicine, pediatrics, obstetrics, neonatology, medical genetics, and teratogen risk counseling. In addition, BDRP publishes the scientific journal, Birth Defects Research. Learn more at http://www.birthdefectsresearch.org. Find BDRP on LinkedIn, Facebook, Twitter and YouTube.
Published Febuary 2020

By Mona Dai, PhD
Student Member Since 2018
This blog is also published on Medium.com and LinkedIn.
Running. If I had to choose just one word to describe my experience pursuing my doctorate, I would choose “running”. Almost every day I’m running from classes to meetings to talks; I’ve never had a week exactly like the one before; that’s why one of my favorite things about student life is the variety.
Before becoming a graduate student, I worked as a research fellow at the Environmental Protection Agency (EPA). During my EPA fellowship, I was inspired by the passion and dedication of each of my co-workers to truly serve the public and challenged myself to fill their shoes one day. I decided to pursue a doctorate degree with the intent to work on research that will support implementation of sound, scientific policy for public health. I hope to become an expert who can help advise and forward responsible policies. That’s why I’ve been so excited to have started my PhD journey.
Although my classes are mostly traditional lectures, most of my class sizes are small, with less than twelve students per class. As a result, questions are encouraged and more than once we have had so much back and forth between professor and students that we’ve had an extra class-dinner provided—to go over the material we weren’t able to cover during a normal session. Every week there are meetings for my lab group to talk about our projects, for my department to discuss general happenings and concerns, and presentations by rotating PhD students on whatever subject of their choice. We give updates on our individual projects and, if a conference is upcoming, listen to and critique fellow group members’ presentations. A number of presentations on non-scientific topics are also sprinkled throughout the semester. For instance, I’ve listened to my classmates lecture passionately about the epic of Gilgamesh and recommend which lesser known national parks deserve a visit. Usually these meetings are popular not only because of the free food (the best way to convince graduate students to do anything), but also because they are lighthearted meetings that help us practice our presentation skills.
Woven in the time between classes and meetings is when I complete most of my research. Since most of my lab group are computer modelers, you can often find us coding away on our computers using a variety of programs: R, Python, Matlab, or Fortran. We’ll be creating maps trying to identify how mercury travels across the globe or creating bioaccumulation models to understand how PFAS biomagnifies within food systems. I really appreciate modeling because I always feel a sense of achievement knowing that I can compare my model to real data to get an intuition of whether I’m coding in the right direction. It’s a great self-check before developing it further to be able to better manage the future. Since I often feel more productive working in a group setting so that we can bounce ideas off each other, we’ll usually work in spurts of a few hours and take periodic breaks to search for snacks.
I’m also extremely grateful for all the social events thrown to nudge graduate students out of their offices. Of course there is always an unending number of inspiring academic speakers giving lectures at the university at any time, but there are also countless opportunities to try something new on campus or to go out and see the city. One Friday afternoon I attended a fresco class at the art museum. Another time I explored the aquarium with my cohort. Yet another weekend I ventured up to New Hampshire for a getaway in the mountains. Our department also has weekly group meditations. I’m also excited for opportunities, such as the one provided through BDRP’s 2020 Howard Garrison Public Affairs Fellowship, to travel off campus and delve into the real policy world. Through the fellowship, I will be traveling to Washington DC to meet with members of Congress to advocate for sustained scientific research. I look forward to learning about and practicing how my voice as a scientist can be shared to promote these issues both now and in my future career.
The biggest problem a graduate student has is not that there is nothing to do—it is that there is too much! However, I think it’s a good problem to have, especially as my journey marches me closer towards my goal of serving the public.
About the Author
 Mona Dai is currently a PhD student at Harvard University in environmental science and engineering working on global pollutants including perfluorinated compounds (PFAS). She previously worked as an ORISE Research Fellow hosted by the US Environmental Protection Agency (US EPA) in the Office of Children's Health Protection. Mona was a recipient of a Society for Birth Defects Research and Prevention Student and Postdoctoral Travel Award for the 2018 Annual Meeting held in Clearwater, Florida, and the 2020 BDRP Howard Garrison Public Affairs Fellowship recipient. To learn about all of the current travel opportunities for students interested in attending the Annual Meeting, please visit https://www.birthdefectsresearch.org/meetings/2020/student-travel.asp
Mona Dai is currently a PhD student at Harvard University in environmental science and engineering working on global pollutants including perfluorinated compounds (PFAS). She previously worked as an ORISE Research Fellow hosted by the US Environmental Protection Agency (US EPA) in the Office of Children's Health Protection. Mona was a recipient of a Society for Birth Defects Research and Prevention Student and Postdoctoral Travel Award for the 2018 Annual Meeting held in Clearwater, Florida, and the 2020 BDRP Howard Garrison Public Affairs Fellowship recipient. To learn about all of the current travel opportunities for students interested in attending the Annual Meeting, please visit https://www.birthdefectsresearch.org/meetings/2020/student-travel.asp
More about the Society for Birth Defects Research and Prevention (BDRP)
To understand and prevent birth defects and disorders of developmental and reproductive origin, BDRP promotes multi-disciplinary research and exchange of ideas; communicates information to health professionals, decision-makers, and the public; and provides education and training.Scientists interested in or already involved in research related to topics mentioned in this blog are encouraged to join BDRP and attend the 60th Annual Meeting June 27–July 1, 2020, the premier source for cutting-edge research and authoritative information related to birth defects and developmentally-mediated disorders. Our members include those specializing in cell and molecular biology, developmental biology and toxicology, reproduction and endocrinology, epidemiology, nutritional biochemistry, and genetics, as well as the clinical disciplines of prenatal medicine, pediatrics, obstetrics, neonatology, medical genetics, and teratogen risk counseling. In addition, BDRP publishes the scientific journal, Birth Defects Research. Learn more at http://www.birthdefectsresearch.org. Find BDRP on LinkedIn, Facebook, Twitter and YouTube.
Published January 2020
By Janet R. Hardy, PhD, MPH, MSc, and Melissa S. Tassinari, PhD, DABT
The social media feed read ‘...Women are under-researched, under-diagnosed, and dying unnecessarily…help close the research gap’. There’s a lot of truth in that statement. Attention to women’s health research has lagged behind men since…well, since the beginning.
But just as every important story has an element of transformation, the period for transforming women’s health is now. And just as the PRGLAC is a critical piece of that transformation, the Society for Birth Defects Research and Prevention (BDRP) and Organization of Teratology Information Specialists (OTIS) have the expertise to play a role in that transformation.
The PRGLAC refers to the Task Force on Research Specific to Pregnant Women and Lactating Women. This Task Force was established in 2017 by the 21st Century Cures Act, a law designed to help speed up medical product development. Managed by the National Institutes of Health (NIH), the PRGLAC is made up of representatives of all Federal agencies with responsibilities for women’s health and research, clinicians, industry and other experts.
In Phase I, PRGLAC was charged with identifying gaps in knowledge and research regarding safe and effective therapies for pregnant women and lactating women. The Task Force conducted four public meetings in 2017 and 2018 and submitted their conclusions to Congress and the Secretary of Health and Human Services in a publicly available report. The report provides 15 specific recommendations to address the areas under the PRGLAC charge (Figure 1). Overall, the report provides thoughtful detail on the magnitude of challenges currently facing research for pregnant women and lactating women. It identifies where data are needed to help inform therapy choices and recognizes that data needs are different for these two populations. Additionally, a central theme emerged from the public discussions: that progress will be made only after a change occurs in long-held cultural assumptions about the participation of pregnant and lactating women in clinical research. Operationally, this would translate into the inclusion of pregnant and lactating women in clinical research or a firm justification for their exclusion. For BDRP and OTIS clinicians and researchers, the recommendations and discussion theme will not be new, but an important platform for conversation has been set.
Figure 1.
Executive Summary, Box 2: Task Force on Research Specific to
Pregnant Women and Lactating Women Recommendations
- Include and integrate pregnant women and lactating women in the clinical research agenda
- Increase the quantity, quality, and timeliness of research on safety and efficacy of therapeutic products used by pregnant women and lactating women
- Expand the workforce of clinicians and research investigators with expertise in obstetric and lactation pharmacology and therapeutics
- Remove regulatory barriers to research in pregnant women
- Create a public awareness campaign to engage the public and health care providers in research on pregnant women and lactating women
- Develop and implement evidence-based communication strategies with health care providers on information relevant to research on pregnant women and lactating women
- Develop separate programs to study therapeutic products used off-patent in pregnant women and lactating women using the National Institute of Health (NIH) Best Pharmaceuticals for Children Act (BPCA) as a model
- Reduce liability to facilitate an evidence base for new therapeutic products that may be used by women who are or may become pregnant and by lactating women
- Implement a proactive approach to protocol development and study design to include pregnant women and lactating women in clinical research
- Develop programs to drive discovery and development of therapeutics and new therapeutic products for conditions specific to pregnant women and lactating women
- Utilize and improve existing resources for data to inform the evidence and provide a foundation for research on pregnant women and lactating women
- Leverage established and support new infrastructures/collaborations to perform research in pregnant women and lactating women
- Optimize registries for pregnancy and lactation
- The Department of Health and Human Services Secretary should consider exercising the authority provided in law to extend the PRGLAC Task Force when its charter expires in March 2019
- Establish an Advisory Committee to monitor and report on implementation of recommendations, updating regulations, and guidance, as applicable, regarding the inclusion of pregnant women and lactating women in clinical research
Source: Task Force on Research Specific to Pregnant Women and Lactating Women; Report to Secretary, Health and Human Services, Congress, September 2018
In May 2019, the PRGLAC re-convened with a two-year mandate and a new charge for Phase II: to develop plans for implementing the recommendations laid out in the Phase I report. The PRGLAC membership was expanded and divided into four working groups, to address the recommendations of the report: WG1 Research and Training; WG2 Regulatory; WG3 Communication and Registries; and WG4 Discovery. Deliberations within each of the four working groups are currently underway and a new report is being drafted.
Members of BDRP and OTIS participated in the Phase I PRGLAC meetings and the Society submitted written comments to the public docket. The report highlighted opportunities for BDRP, OTIS and their respective members to take a more active role as PRGLAC progressed into Phase II activities. Following the annual BDRP meeting in San Diego, June 2019, a joint BDRP-OTIS PRGLAC Working Group was formed, including members from both societies, which we are co-chairing along with Beth Conover. The charge of this working group is to:
- Influence the PRGLAC discussions and in so doing, highlight both societies and make a positive impact on research specific to pregnant and lactating women;
- assist in Implementation of the PRGLAC recommendations; and
- support Communication of PRGLAC recommendations to a larger community.
The main BDRP-OTIS PRGLAC Working Group activities thus far include:
- The group has produced a document of thoughtfully constructed comments in response to the PRGLAC Phase I report. These were submitted to the PRGLAC and made publicly available. Additionally, a summary of the comments was presented by Janet Hardy, representing both societies, during the meeting’s public discussion period, of which a recorded webcast is available.
- The group also developed a member survey based on the four topics of PRGLAC working groups. The purpose was to identify BDRP and OTIS members whose research and program work could be directly relevant and/or helpful to the PRGLAC. The preliminary results are currently being analyzed. The results may also better inform the membership of both societies regarding work conducted by members, expertise of members, and future priorities.
The efforts of PRGLAC provide a fresh conversation to address long-standing issues to provide evidence-based information for the treatment of pregnant and lactating women. The PRGLAC stands to impact many aspects of BDRP and OTIS members’ work, and to impact the lives of pregnant women and lactating women. Similarly, BDRP and OTIS have the history, and members have the depth of experience to meaningfully impact PRGLAC’s discussions and work. It’s a partnership that is imperative to nourish.
So, fellow BDRP and OTIS members: The populations and work we have been passionate about throughout our careers are in the spotlight…for a short period. Where PRGLAC is a call to action…for us, it’s a call to pay attention and to engage in the transformation.
About the Authors
Janet Hardy, PhD, MSc, MPH is Assistant Professor (affiliate) at U. South Florida College of Public Health, and Consultant, providing expertise-driven research & programmatic consulting to nonprofit, for-profit & legal organizations in 1) pharmacoepidemiology; and 2) maternal-child health research/programming in global low-resource settings. Dr. Hardy has been a BDRP member since 2005 and has served on the Education, Program and Science Committees.
Melissa S Tassinari, PhD, DABT has been a member of BDRP since 1978 and has served on Council and as Society President [2005-06]. She is a developmental toxicologist with experience in regulatory policy and is now retired after a career focused on maternal and child health at Pfizer and as a Senior Clinical Advisor in the Division of Pediatric and Maternal Health at the FDA.
More about the Society for Birth Defects Research and Prevention (BDRP)
The Society for Birth Defects Research and Prevention (BDRP) is a multidisciplinary society of scientists from a variety of disciplines including researchers, clinicians, epidemiologists, and public health professionals from academia, government, and industry who study birth defects, reproduction, and disorders of developmental origin. Our members include those specializing in cell and molecular biology, developmental biology and toxicology, reproduction and endocrinology, epidemiology, nutritional biochemistry, and genetics, as well as the clinical disciplines of prenatal medicine, pediatrics, obstetrics, neonatology, medical genetics, and teratogen risk counselling.
BDRP convenes an annual scientific meeting annually where members and others share their research, gain new knowledge and continuing education, mentor the next generation of researchers in the field, and network. The Society publishes the peer-reviewed scientific journal, Birth Defects Research. Learn more at http://www.birthdefectsresearch.org. Find BDRP on LinkedIn, Facebook, Twitter and YouTube.
2019
Published October 2019

By Christine Perdan Curran, Northern Kentucky University
President, Society for Birth Defects Research and Prevention
The Teratology Society is proud to announce a new name and logo to celebrate its long history and dedication to protecting the world’s most vulnerable population through the combined efforts of scientists and clinicians. As the Society approaches its 60thAnniversary, it’s a tribute to the important work we do and clearly communicates the impact and value of our scientific efforts.
When my mother-in-law told me that my research was too complicated to understand, I made it simple. I told her we’re trying to “save the babies.”
When a colleague asked me what we do, I answered “the most important work on Earth-protecting the next generation.”
As we considered a change to our name and logo, it was clear that to improve Society recognition and communicate effectively, we needed to simplify our message, much as I had done with my mother-in-law and colleague. The conclusion was the result of careful, deliberative work - great ideas were shared, tried, tested, and tested again.
The result was a name that clearly describes our work and a logo that focuses on the positive results of our work: a healthy child supported and swaddled by the protective arms of scientists and clinicians. Our history is honored as our new name rests solidly on our founders’ legacy.
From the early 1960s when the Society founders tackled important questions about chromosomal abnormalities and the horrors of thalidomide, our collaborative approach has led to scientific breakthroughs that informed public policy and clinical practice. Examples include the recognition of fetal alcohol syndrome, warnings about excessive Vitamin A use during pregnancy as well as the critical importance of folate in preventing neural tube defects.
We still have a great deal of important work to do collectively and collaboratively. One in 33 U.S. babies is born with a birth defect. Worldwide, more than 8 million babies are affected each year. Fortunately, our transdisciplinary and transformative science has positioned BDRP well to address these continuing concerns. Examples include innovative research on epigenetics, single cell transcriptomics, and computational methods and fast action by alert clinicians to respond to the risks of Zika virus during pregnancy and neonatal abstinence syndrome in the wake of the opioid epidemic.
I encourage current members and future members to learn more as we celebrate our 60thAnniversary in Charleston SC June 27 to July 1st 2020 at our Annual Meeting “Honoring Teratology’s Roots, Growing a Healthier Future.”
We strongly support our trainees with travel awards, mentoring and education resources, such as our Primer, and offer professional development to all.
I welcome you to join us in the most important and most rewarding work on Earth.
About the Author
Christine Perdan Curran, PhD, is a professor in the Department of Biological Sciences at Northern Kentucky University. She teaches and conducts research in the areas of developmental neurotoxicology, gene-environment interactions, persistent organic pollutants, as well as traffic-related air pollution, microbiome, and energy drink toxicity.
More about the Society for Birth Defects Research and Prevention (BDRP)
To understand and prevent birth defects and disorders of developmental and reproductive origin, BDRP promotes multi-disciplinary research and exchange of ideas; communicates information to health professionals, decision-makers, and the public; and provides education and training.
Scientists interested in or already involved in research related to topics mentioned in this blog are encouraged to join BDRP and attend the 60th Annual Meeting June 27–July 1, 2020, the premier source for cutting-edge research and authoritative information related to birth defects and developmentally-mediated disorders. Our members include those specializing in cell and molecular biology, developmental biology and toxicology, reproduction and endocrinology, epidemiology, nutritional biochemistry, and genetics, as well as the clinical disciplines of prenatal medicine, pediatrics, obstetrics, neonatology, medical genetics, and teratogen risk counseling. In addition, BDRP publishes the scientific journal, Birth Defects Research. Learn more at www.birthdefectsresearch.org. Find BDRP on LinkedIn, Facebook, Twitter and YouTube.

By Suzan Carmichael, PhD, MS
This blog is also published on Medium.com and Linkedin.
Maternal mortality refers to maternal deaths that are related to pregnancy and occur during pregnancy or soon after giving birth. Did you know that in the US, our rate of maternal mortality is higher than any other high-income country? This is not acceptable. This already embarrassingly high rate is on the rise–maternal deaths are actually increasing in the U.S. Severe maternal morbidities–conditions that put women most at risk of death, but are 50–100 times more common than death–are also increasing. Unacceptable. In addition, maternal death is 3–4 times more common among black women than white women. Again, unacceptable.
Be Part of the Solution
For many years, I have conducted research about the impact of maternal health on the developing fetus-much of it has been about birth defects. As these disturbing trends in maternal health have emerged, I've become more interested in understanding why they have occurred and how we can reverse them. It's especially interesting to me that more than half of all maternal deaths and severe maternal morbidity could probably be prevented through better care-so, a potential solution does exist! Last year, Linda Roberts, PhD, chair of the Teratology Society Communications Coordination Committee, and Mary Alice Smith, PhD, a Teratology Society Past President, felt this topic was important to include in the annual meeting. They contacted me to find out if I knew anyone working on maternal mortality in California, given that they had heard about its unique trends, and it’s where I live. Did you know California is the only state where maternal mortality has actually declined in recent years? There is something vital to learn from the Golden State. Anyway, I told them that I was actually very interested in this topic myself, and that I was beginning to focus my own research in this area.
That's how I became a partner in developing a critical symposium at the Teratology Society’s Annual Meeting in San Diego, CA June 22–26, which I am co-Chairing with Dr. Smith. I hadn't planned to present this work to my ‘birth defects’ colleagues, but one of the wonderful things about this Society is its interest in a broad array of issues that contribute to fetal development and child health.
Understanding the “Why” While Learning from Others
Before we can figure out a solution to the disturbing trends of maternal mortality in this country, we must first better understand why it’s happening. The first two speakers at the “Maternal Morbidity and Mortality: Causes, Disparities, and Solutions” symposium will examine the reasons that the U.S. lags behind other nations in reducing maternal deaths and potential explanations for racial-ethnic disparities. The third speaker will provide examples of how state programs are attempting to improve maternal health, including a hospital quality care collaborative in California-which will help us understand how the reductions in maternal morality have been achieved here.
The symposium will continue with two presentations that examine cardiovascular and hypertensive disorders during pregnancy, including their impact on maternal health and fetal development. These conditions have lifelong impacts on the health of mothers and infants and are important contributors to maternal mortality. In fact, cardiovascular conditions are the number one cause of maternal death.
There is much to understand about maternal morbidity and only time will tell if we can reverse trends quickly or not. However, one thing is for certain-we all agree that whatever we can do to improve the mother's health will have obvious, and multi-faceted, positive impacts for both the mother and the baby. I hope you’ll consider being part of that impact. See you at #TS2019 in San Diego.
About the Author
Suzan Carmichael, PhD, MS, is a perinatal epidemiologist and Professor in the Departments of Pediatrics and Obstetrics and Gynecology at the Stanford University School of Medicine. She has been a member of the Teratology Society since 1999 and has served on the Program and Public Affairs Committees. She was the recipient of the Teratology Society’s F. Clarke Fraser New Investigator Award in 2005.
About the Teratology Society
To understand and prevent birth defects and disorders of developmental and reproductive origin, the Teratology Society promotes multi-disciplinary research and exchange of ideas; communicates information to health professionals, decision-makers, and the public; and provides education and training.
Scientists interested in or already involved in research related to topics mentioned in this blog are encouraged to join the Teratology Society and attend the 59th Annual Meeting June 22–26, 2019, the premier source for cutting-edge research and authoritative information related to birth defects and developmentally-mediated disorders. Teratology Society members include those specializing in cell and molecular biology, developmental biology and toxicology, reproduction and endocrinology, epidemiology, nutritional biochemistry, and genetics, as well as the clinical disciplines of prenatal medicine, pediatrics, obstetrics, neonatology, medical genetics, and teratogen risk counseling. In addition, the Teratology Society publishes the scientific journal, Birth Defects Research. Learn more at www.Teratology.org. Find the Teratology Society on LinkedIn, Facebook, and Twitter.
Published March 2019

By Lauren M. Walker, BS
This blog is also available on Medium.com and LinkedIn.
I focused on the soft click-clack sound of people’s shoes on the marble floor to redirect my nervous energy. “We have about 20 minutes before our first meeting,” Sally Schwettmann, our Federation of American Societies for Experimental Biology (FASEB) staff guide, said, “Let’s hang out outside until 5 minutes before.” We were standing outside the office of Congressman Mark Takano—U.S. Representative for California’s 41st district, my district—on Capitol Hill. Through a Teratology Society-funded FASEB Howard Garrison Public Affairs Fellowship, I was able to join a coalition of 58 scientists from 30 states to participate in FASEB’s 2019 Capitol Hill Day. Capitol Hill Day’s objective is to remind our representatives and senators of the important role that biomedical research plays in the lives of everyday Americans, and how critical stable and sufficient funding from key agencies like the NSF and NIH is in supporting research discoveries and the future of STEM (science, technology, engineering and math).
FASEB is an association of 29 different scientific societies—including The Teratology Society—that serves to promote research and education in the biomedical sciences. FASEB encourages scientists to get involved with their communities and communicate the importance of their research. Each year, FASEB organizes a Capitol Hill Day to encourage face-to-face meetings of scientists and policy makers. These meetings are critical as they introduce to policymakers the people behind the research and allow a sharing of real-life impacts of research funding decisions. FASEB hosts an advocacy training dinner the evening before to prepare participants for the Hill. During the dinner, I was delighted to practice my talking points with Dr. Howard Garrison (pictured with me above), the scientist after whom my fellowship is named. Dr. Garrison recently retired after leading the FASEB Office of Public Affairs for over 30 years. Over dinner, he shared his previous Hill Day experiences and advised me to stay engaged and be persistent. “Time and people do change,” he said, “and you can be a part of that.”
During our meeting with Congressman Takano’s staff the next morning, I relayed how my project’s funding was cut just as I was preparing for my Advancement to Candidacy exam. Losing that funding meant building up a new project from scratch, and hoping that my lab could secure enough funding to see the project to completion and culminating in my doctoral degree. After a year, my lab successfully secured an NIH grant. I shared with the staff what it felt like to be anxious about the future of both my education and career. I stressed that both depend on the quantity and quality of my work which, in turn, depend on research funding. It may have been chilly outside that morning in Washington, DC, but my passion for securing funding kept me warm.
It may have been chilly outside, but my passion for securing funding kept me warm.
I walked out of that first meeting feeling exhilarated and valued. I couldn’t wait for our next meeting! For the rest of the day, my co-delegate, Dr. Ralph Bradshaw of the University of California, Irvine, and I teamed up to compile several examples of the real, human impacts of research funding that our representatives could use to support their arguments and influence decisions on increasing resources for biomedical research. While our California representatives are already quite supportive of NSF and NIH research funding, Dr. Bradshaw was quick to point out that “it’s always good to visit with friends.” Showing up to supportive policymakers’ offices to thank them for their support and reiterate the importance of reliable research funding shows that scientists are paying attention to the Hill. Moreover, it reminds policymakers that scientists are invested in the future of science funding.
As recent years have demonstrated, biomedical research funding is not static. We must speak up or risk being forgotten. Without our voices, funding could be further constrained in future years. It’s especially critical that policymakers hear from up-and-coming scientists—graduate students and early career scientists who are paving the way for the future of scientific research. The ultimate goal of Capitol Hill Day is to help scientists establish long-term relationships with their representatives’ offices through regular interaction with policymakers. If you’re already a science advocate, thank you for your contributions. If you haven’t considered becoming a science advocate yet, please do. It’s well-worth it, more influential, and less terrifying than you may think. Participating in science advocacy events in your community or through social media calls-to-action are great ways to get started. If logistics allow, also consider taking part in a future FASEB Capitol Hill Day. It’s a life-changing experience that I certainly can’t wait to participate in again.
About the Author
Lauren Walker is a PhD Candidate studying environmental toxicology at the University of California, Riverside.
About the Teratology Society
To understand and prevent birth defects and disorders of developmental and reproductive origin, the Teratology Society promotes multi-disciplinary research and exchange of ideas; communicates information to health professionals, decision-makers, and the public; and provides education and training. Scientists interested in or already involved in research related to topics mentioned in this blog are encouraged to join the Teratology Society and attend the 59th Annual Meeting June 22–26, 2019, the premier source for cutting-edge research and authoritative information related to birth defects and developmentally-mediated disorders. Teratology Society members include those specializing in cell anad molecular biology, developmental biology and toxicology, reproduction and endocrinology, epidemiology, nutritional biochemistry, and genetics, as well as the clinical disciplines of prenatal medicine, pediatrics, obstetrics, neonatology, medical genetics, and teratogen risk counseling. In addition, the Teratology Society publishes the scientific journal, Birth Defects Research. Learn more at www.Teratology.org. Find the Teratology Society on LinkedIn, Facebook, and Twitter.
Published March 2019
By Marcia L. Feldkamp, PA, PhD & Vijaya Kancherla, MS, PhD
This blog is also available Medium.com and LinkedIn. Please read and share!
March 3rd marks World Birth Defects Day. Birth defects are a daily reality for many families. The stigma associated with birth defects is a world-wide issue. At the same time, people with birth defects have independently excelled in many fronts with their resolve and persistence. As scientists in the field of birth defects research, we are on a constant quest to learn more about birth defects: what causes them, how to track and prevent them, and how to best improve the lives of those born with birth defects. The momentum we have now for birth defects is phenomenal. This World Birth Defects Day, our focus is on how to alleviate the global burden of preventable birth defects in the 21st Century. Birth defects prevention is still very much a critical public health topic—one that’s not often prioritized as much as other childhood health issues.
Birth defects can manifest in a variety of forms. Defects can be due to an abnormal formation of the embryo leading to structural changes at birth. They can be present before birth, at birth, or can be diagnosed later in life. Some defects can be easily visible, while others can occur internally (for example, a heart defect). Birth defects occur mostly during the first three months of pregnancy (first trimester), when the organs and tissues form. Some defects can include abnormality of function, such as metabolic conditions. Environmental exposures in early pregnancy that may increase the risk of birth defects include chemicals and toxins, medications, and infections. Other issues such as poor maternal nutrition, risky health behaviors (e.g., smoking cigarettes, alcohol abuse, recreational drugs), and/or not receiving preventive vaccines are also known to increase the likelihood of birth defects. Some of these exposures can even impact a human fetus during the later stages of pregnancy (e.g., the brain continues to develop throughout pregnancy and is vulnerable to harmful exposures for the entire pregnancy.) Birth defects can also be caused by genetic or chromosomal abnormalities (such as trisomy 21, also known as Down syndrome). An exposure to a single harmful agent may cause a birth defect, though it is thought that several birth defects are likely due to a combination of environmental and genetic factors. Still, we have yet to determine the exact cause for many defects.
On this 5th anniversary of World Birth Defects Day we want to promote awareness about birth defects and to encourage prevention wherever possible. In this spirit, and as birth defects researchers, we want to bring birth defects to the forefront of the discussion on maternal and child health issues. Birth defects cannot be under-prioritized or ignored; they are common, costly, and critical.
- Common: March of Dimes Global Report on Birth Defects estimates that almost 8 million children (6% of total births worldwide) are born with a serious birth defect each year. This figure does not include those fetuses that are stillborn or result in a spontaneous or elective pregnancy termination due to a birth defect. It is important to understand that the global burden number is only an estimate, and the true burden is expected to be much higher than what is estimated. The most common birth defects are heart defects, neural tube defects, and Down syndrome.
- Costly: The costs to care for a child vary by country. In the USA, it has been estimated that the 2013 hospitalization costs for people with birth defects was 23 billion dollars. This estimate does not include the added costs for specialized care, parental loss of work to care for a child, or the emotional costs on all members of a family. With improved care in developed countries, the lives of infants and children have been extended well into adulthood, increasing the overall lifetime costs of caring for someone born with a birth defect.
- Critical: Some birth defects increase the risk of death during infancy and early childhood. For example, the WHO estimated that each year, over 300,000 babies die within 4 weeks of birth due to birth defects; and 17–43% of all deaths in children under age 1 year could be attributed to birth defects. Birth defects are also associated with chronic illness and lifelong disability. Overall, they pose significant burden on not only those affected but their families and health care systems.
The true global burden of birth defects is unknown. Once a baby is born with a birth defect, the family must accept the responsibility of caring for their child, seek appropriate medical care, and care for their other children and family members. Society in many instances does not provide necessary support and opportunities for those with birth defects. Care-related issues persist throughout life, and this becomes even more evident as we see that more and more children with birth defects are successfully reaching adulthood. Available medical care and the daily care of a child or an adult with a birth defect will be very different if a family resides in a developed versus a developing country. To this day, many children born with a birth defect are shunned in developing countries due to lack of awareness, political will, or other support systems.
There are still major gaps in birth defects surveillance and research in low- and middle-income countries
Many countries in the developing world do not have an infra-structure to count pregnancies affected by birth defects in the population. Unfortunately, birth defects tend to be more common in these same regions with low education, poverty, lack of prenatal care, undernutrition, low rates of immunization, consanguinity (i.e., where parents are blood-related), and persistent high-risk behaviors during pregnancy. Governments in these countries are not motivated to invest in birth defects surveillance as they do not see it as a priority. Birth defects are lost from the sight of policy makers as they end in stillbirths, or affected babies die soon after birth due to lack of early surgical and medical care. Research on birth defects is also limited in low- and middle-income countries due to the same reasons mentioned above. The recent concept of “triple surveillance”, where there is an investment in not only measuring the occurrence of birth defects in the population, but adding on surveillance of associated factors (e.g., nutritional deficiency in women), and health outcomes (e.g., mortality associated with a birth defect) can lead to better outcomes in birth defects.
Prevention requires quality research endeavors and effective healthcare policies
There is a need for high-quality birth defects research to investigate suspected factors that may be associated with specific birth defects. Primary prevention, meaning preventing birth defects before they even occur, requires knowing the factors that can cause or reduce birth defects, and understanding how to best address these factors and implement prevention programs in the population. Understanding whether a prevention activity is of value in a given population requires regular population-based surveillance of birth defects. Countries also will need strict implementation and evaluation of the prevention programs. Where there are effective evidence-based healthcare policies along with financial and regulatory support from the governments, it is shown that the burden of birth defects is reduced.
Some birth defects can be prevented immediately with available interventions
Of the many birth defects that occur globally, some are immediately preventable because we already know how to prevent them. We have proven and cost-effective solutions that can prevent major and common birth defects. For example, congenital rubella syndrome can be prevented by giving girls and young women rubella vaccine before they are pregnant. Fortifying staple foods with folic acid and iodine will also prevent neurological birth defects. Educating women about the risks of consuming alcohol during pregnancy and providing prenatal counseling can reduce fetal alcohol syndrome in their babies. Some cases of Down syndrome can be prevented by societies supporting women to plan their pregnancy at a younger age in developed countries and improving prenatal screening and counseling. Limiting the use of teratogenic medications (that is, medications that are associated with birth defects) during pregnancy is another important way to intervene. Screening and treating maternal health conditions like diabetes, hypertension, and infections, before and during early pregnancy will also reduce the occurrence of birth defects.
Monitoring trends in global birth defects prevalence offers an informed look at current risks
The presence of birth defects in a community is a dynamic phenomenon to some extent. Countries need to constantly track birth defects to establish a baseline, develop public health interventions so as to prevent them when possible, and provide care and services to those affected. As we gain more information about the risk and preventive factors, birth defect trends change. Over recent decades, as more women in developed countries are delaying their pregnancies, we are noticing an increase in the prevalence of babies born with Down syndrome. Changes in environment and infectious agents (e.g., mosquitoes) has recently resulted in an epidemic of Zika-related microcephaly. On the upside, as more and more countries fortify staple foods or grains with folic acid, iodine, and other important nutrients, we are seeing a decrease in birth defects associated with maternal nutritional deficiencies (e.g., folic acid fortification of wheat and maize products has resulted in a marked reduction of neural tube defects in many countries). With the world in constant flux, observing changes in global trends in birth defects, and taking timely action to address issues, is both a public health challenge and a necessity.
In most developed countries, ultrasound and maternal blood and serum screening are commonly used to perform prenatal screenings for abnormal development. Prenatal screening can pick up birth defects related to genetic or chromosomal abnormalities, or those such as heart and neural tube defects. Abnormal screening results are followed up with confirmatory diagnostic procedures like chorionic villus sampling and amniocentesis. Many low- and middle-income countries, however, lack funds and facilities to offer such birth defect screening or diagnosis prenatally. This discrepancy needs to be addressed to close the global disparity on birth defects occurrence. Prenatal screening helps parents/families to prepare for the birth of their baby that may have birth defects and associated disabilities later in life.
Care and advocacy for those living with birth defects should be prioritized
Care and rehabilitation for those with birth defects is often ignored. Quality of life for those with birth defects is largely dependent on the severity of the defect. Economic status of the countries also plays a role in the quality of care. Disparities in care and quality of life of those with birth defects a serious concern worldwide. Many babies with birth defects die soon after birth in developing countries due to lack of needed medical care or resources. Those babies who survive after birth are sometimes mistreated and stigmatized by the community. Many children born with birth defects do not receive education, sufficient medical care, or gain employment. Global awareness for birth defects and the needs of those with birth defects is still lacking. With adequate support and care, people with birth defects can be successful and fully contributing members of the society. Countries should strive towards making birth defects prevention a global priority, while not forgetting that those individuals with birth defects are an integral part of the society.
In conclusion, this #WorldBDday March 3 and every day, we and our colleagues would like to encourage everyone to educate the general public, local politicians, and government agencies about birth defects. The louder we are as we communicate #ManyBirthDefects1Voice, the greater potential we have in reaching out to policy makers and systems that can improve outcomes for birth defects.
About the Authors
Marcia Feldkamp, PA, PhD, developed an interest in birth defects epidemiology while working as a physician assistant and caring for women of reproductive age. Due to the limitations of existing data in Utah, Dr. Feldkamp led the efforts to develop a clinically based birth defects surveillance system in Utah to provide necessary data to conduct research to improve our understanding of pathogenesis and etiology. Dr. Feldkamp is a Research Professor in the Division of Medical Genetics, Department of Pediatrics at the University of Utah.
Vijaya Kancherla, MS, PhD, completed her PhD in epidemiology at the University of Iowa and then went on to the Pierre Decouflé Post-Doctoral Fellowship at the CDC National Center on Birth Defects and Developmental Disabilities. Currently Dr. Kancherla is a Research Assistant Professor at the Rollins School of Public Health at Emory University. Dr. Kancherla was introduced to the study of birth defects early in her doctoral program and instantly recognized the need and impact of this field.
About the Teratology Society
To understand and prevent birth defects and disorders of developmental and reproductive origin, the Teratology Society promotes multi-disciplinary research and exchange of ideas; communicates information to health professionals, decision-makers, and the public; and provides education and training. Scientists interested in or already involved in research related to topics mentioned in this blog are encouraged to join the Teratology Society and attend the 59th Annual Meeting June 22–26, 2019, the premier source for cutting-edge research and authoritative information related to birth defects and developmentally-mediated disorders. Teratology Society members include those specializing in cell and molecular biology, developmental biology and toxicology, reproduction and endocrinology, epidemiology, nutritional biochemistry, and genetics, as well as the clinical disciplines of prenatal medicine, pediatrics, obstetrics, neonatology, medical genetics, and teratogen risk counseling. In addition, the Teratology Society publishes the scientific journal, Birth Defects Research. Learn more at www.Teratology.org. Find the Teratology Society on LinkedIn, Facebook, and Twitter.

By Vijaya Kancherla, MS, PhD
Read/Comment/Share on: Medium.com or LinkedIn
“My name is Amanda Mateus. I am the mother of three sons and one daughter. My youngest son has Spina Bifida.” Amanda is not alone. In fact, she’s joined by countless families around the world dealing with the after-effects of neural tube defects (NTD), like spina bifida and anencephaly, which are largely preventable. “I believe that advocating for better birth defect prevention research is not devaluing in any way those who have a birth defect,” writes Mateus. “If it weren’t for research, knowledge, and implementations, many people who now live with a birth defect would not be alive today.” It’s the core reason behind the Teratology Society’s passion to reduce birth defects worldwide and it’s what drove the latest publication of a position statement on the surveillance and methods for prevalence estimation of NTDs in a recent issue of the Birth Defects Research journal. Considering that the most prestigious society in this field has only published official positions on a handful of other birth defects-related issues, this is significant. Here’s why it’s chosen to focus on the critical issue of NTDs.
NTDs are some of the most common types of birth defects occurring worldwide.
NTDs occur when the neural tube, which forms the brain and spinal cord in the fetus, is not closed completely. NTDs form around the 4th week of gestation, when most women are unaware of their pregnancies. There are different kinds of NTDs depending on the level where the neural tube closure fails. They can be easily identified during pregnancy and at birth. Anencephaly, spina bifida and encephalocele are common forms of NTDs. Babies born with spina bifida face a high risk of death in early childhood and suffer lifelong disability and health issues. Babies with anencephaly die soon after birth. In addition, many anencephaly and spina bifida‐affected pregnancies result in stillbirths or are electively terminated after prenatal diagnosis, depending on availability of prenatal diagnostic services and existing elective termination policies.
Anencephaly and spina bifida affect pregnancies worldwide and are largely preventable when the mother takes folic acid before and during early pregnancy.
Birth defects tracking or surveillance systems measure the occurrence of NTDs in the society. A good surveillance system is designed to track all pregnancy outcomes. Miscarriages, elective terminations after a prenatal diagnosis of NTD, and stillbirths that are associated with NTD have to be counted to understand the total number of NTDs occurring in the population. Such systems give us a complete picture of NTDs. But many countries only count NTDs among babies that are born alive, which is like looking at the tip of an iceberg. Good birth defects surveillance systems that count all pregnancies rarely exist in developing countries, where NTDs are expected to affect a high number of pregnancies because mothers lack enough folic acid in their diets for a healthy pregnancy. There are many NTD cases that are missed by not counting all affected pregnancies, and are therefore invisible. Incomplete surveillance has severe consequences, as it thwarts prevention efforts.
The subject of the Teratology Society position statement was conceived by Dr. Robert J. Berry (Emory University), as public health action for NTD prevention has been long hindered by a lack of, or inaccuracies in, existing surveillance in many countries where the prevalence of NTD-affected pregnancies is often especially high. In December 2016, Drs. Berry and Vijaya Kancherla (Emory Unsdiversity) were involved in the Technical Consultation on Folate Status in Women and Neural Tube Defect Prevention, and contributed to several publications on current knowledge, gaps, and challenges in the occurrence of spina bifida and anencephaly globally. Their contribution was integrated into a series of publications in the New York Academy of Sciences in 2018.
During the 57th Annual Meeting of the Teratology Society in Denver in 2017, a symposium focused on informing the meeting participants about the continuing issue of incomplete surveillance and prevalence estimation procedures for the global burden of congenital disorders. At the end of the symposium one of the audience members asked what the Society could do to address the issue. That question became the seed for the position statement. Drs. Vijaya Kancherla and Robert Berry joined with two other interested birth defects surveillance and epidemiology experts, Suzan Carmichael (Stanford University) and Marcia Feldkamp (University of Utah) and drafted the position statement to promote better surveillance of NTD globally. The position statement was endorsed by the Society in 2018.
The statement’s nuts and bolts for building a better surveillance system.
In the position statement, the Teratology Society acknowledges the value of accurate measurement of all cases of NTDs in a population, including all pregnancy outcomes, not just live births. The need for comprehensive surveillance is presented as an essential investment for accelerating the prevention of NTDs. The issue raised is that governments in low and middle income countries where there are a high number of NTD cases that result in stillbirths, and in countries where NTDs are electively terminated soon after prenatal diagnosis, rarely can provide a complete assessment of burden of these birth defects. The impact of NTD-related pregnancy losses among families and societies cannot be ignored! In the position paper, the Society addresses this issue, and recommends approaches for standardizing and improving NTD prevalence estimation that allow capturing all cases and estimating total prevalence. The statement also promotes population-based surveillance methods that utilize multiple data sources and standard data collection protocols to study all pregnancies, as well as neonatal mortality associated with NTDs. The statement promotes assessment of all NTD phenotypes (i.e., anencephaly, spina bifida, encephalocele, and other types of NTDs) and those co-occurring with other major birth defects, or as part of a syndrome, to understand their total prevalence.
A global call for help.
The Teratology Society calls for a collective effort by countries to engage in robust surveillance of NTDs globally by following standard procedures. Partner organizations that have endorsed the statement include the Organization of Teratology Information Specialists (OTIS)/MotherToBaby, Developmental Neurotoxicology Society (DNTS), European Teratology Society (ETS), the National Birth Defects Prevention Network (NBDPN), and the Board and Scientific Committee of the European Network of Teratology Information Services (ENTIS).
“I can’t pretend that we don’t struggle sometimes.”
Amanda hopes others join the global call as she explains the challenges her family faces with her son’s spina bifida. “The physical, emotional, and even financial burdens can be overwhelming at times. We understand that everyone faces challenges, but we want to recognize that there can be unique challenges affecting people with disabilities and their families. We share our story with the hopes that someone will find comfort, will know that they are not alone, and to encourage research that will continue to improve the lives of everyone.”
Together, during January Birth Defects Prevention Month and every month, we can prevent birth defects like NTDs. So who else is ready to join the movement and do what’s #Best4YouBest4Baby?
About the Author
Vijaya Kancherla, MS, PhD, completed her PhD in epidemiology at the University of Iowa and then went on to the Pierre Decouflé Post-Doctoral Fellowship at the CDC National Center on Birth Defects and Developmental Disabilities. Currently Dr. Kancherla is a Research Assistant Professor at the Rollins School of Public Health at Emory University. Dr. Kancherla was introduced to the study of birth defects early in her doctoral program and instantly recognized the need and impact of this field.
About the Teratology Society
To understand and prevent birth defects and disorders of developmental and reproductive origin, the Teratology Society promotes multi-disciplinary research and exchange of ideas; communicates information to health professionals, decision-makers, and the public; and provides education and training. Scientists interested in or already involved in research related to topics mentioned in this blog are encouraged to join the Teratology Society and attend the 59th Annual Meeting June 22–26, 2019, the premier source for cutting-edge research and authoritative information related to birth defects and developmentally-mediated disorders. Teratology Society members include those specializing in cell and molecular biology, developmental biology and toxicology, reproduction and endocrinology, epidemiology, nutritional biochemistry, and genetics, as well as the clinical disciplines of prenatal medicine, pediatrics, obstetrics, neonatology, medical genetics, and teratogen risk counseling. In addition, the Teratology Society publishes the scientific journal, Birth Defects Research. Learn more at www.Teratology.org. Find the Teratology Society on LinkedIn, Facebook, and Twitter.
By Dana Shuey, PhD, DABT
Teratology Society President
In November I had the opportunity to attend a Capitol Hill briefing entitled “The Next Frontier: Research in Pregnant Women and Breastfeeding Women.” The session was sponsored by the Society for Maternal-Fetal Medicine and co-sponsored by other organizations committed to women’s and prenatal health. The intent of the session was to advocate for resources and guidance to break down current barriers to conducting research in pregnant and lactating women. There were presentations from government, industry and academia discussing the current state of research, as well as ongoing challenges, and impact on clinical practice. The event was standing room only!
I came away from the session with two very strong messages involving the birth defects research field that seem incredibly appropriate to discuss now during anuary’s Birth Defects Prevention Month:
1) It is time we stop protecting pregnant and lactating women from research, and protect them through research.
Clinical research in pregnancy and lactation is helping to identify risk factors for high risk pregnancies, including the increasing incidence of maternal mortality and morbidity in the US, leading to important changes in clinical practice guidelines, including elimination of ineffective and often costly practices.
2) Sick women get pregnant, and pregnant women get sick.
When I was having my children 20+ years ago, prevailing medical practice was to avoid any medication exposure, with the exception of prenatal vitamins, unless absolutely critical. However, since then it has been increasingly recognized that many illnesses and diseases carry a significant risk to both the mother and fetus when left untreated and need to be effectively managed during pregnancy. Availability of robust information on the safe and effective use of medicines during pregnancy and lactation is generally lacking, and clinical information is needed to inform clinicians regarding appropriate medication use.
While the briefing was primarily focused on the need for clinical research to support clinical practice guidelines for high risk pregnancies, I was reminded that the same messages certainly apply to birth defects research and prevention. Only through rigorous, multidisciplinary research will we confidently identify risk factors (both genetic and environmental), causes and mechanisms for birth defects and developmental disabilities, with the ultimate goal of prevention.
I have been working in the field of teratology, toxicology and pharmaceutical development for more than 20 years. I was first drawn to birth defects research based on pure fascination with the amazing complexity and orchestration of embryo-fetal development. However, when I joined the Teratology Society as a student, I immediately recognized the strength in the coming together of multidisciplinary expertise with a common goal of understanding and prevention of birth defects. I have remained active in this field and in the Society, even seeking leadership opportunities, with a continued desire to contribute to these activities, build these relationships and to facilitate these interactions to make a difference in the health of mothers and babies.
The importance of a multidisciplinary approach to the understanding and prevention of birth defects is clearly illustrated in several pivotal examples. Fetal alcohol spectrum disorders (FASD) is an important example where experimental data together with rigorous clinical observations, have collectively contributed to a comprehensive understanding of its pathogenesis. Development of an animal model which demonstrates many of the physical features of fetal alcohol syndrome has provided critical information regarding critical period(s), doses, target cell populations, genetic and molecular pathways, and detailed outcomes. Experimental research continues to contribute new understanding regarding mechanisms, neurobehavioral outcomes, genetic susceptibilities and gene-ethanol interactions, which will ultimately translate to a better understanding of clinical risk factors, management and prevention. Recent advances in research and our understanding of FASD will be highlighted in a special issue of our Society journal Birth Defects Research, forthcoming in 2019.
Another example is the recognition of folic acid’s critical role in development, as well as the demonstration of folic acid’s ability to prevent neural tube defects and other congenital anomalies. These discoveries emerged from both experimental and epidemiological studies and has led to policies requiring standard fortification of food in multiple countries. The role of folate and other nutrients in normal and abnormal development, as well as in prevention of birth defects remains a very active area of research. Notably, emerging data on perinatal choline supplementation and neurodevelopmental disorders will be presented in a symposium at the 2019 Teratology Society Annual Meeting.
The Teratology Society, as a multidisciplinary scientific organization is strongly positioned to identify emerging issues and to develop collaborative, translational research strategies to address them.
To leverage the multidisciplinary strength of the Teratology Society, the first annual Multidisciplinary Research Needs Workshop was held at the 2018 Annual Meeting. Participants discussed four emerging issues with research gaps that could benefit from multidisciplinary research. Details and recommendations from the workshop will be issued in a forthcoming workshop summary. Topics and key messages are briefly summarized below:
The Role of Epigenetics in Developmental Origins of Disease
- How the environment (exposures) can influence imprinting preconception and during development.
- The role of obesity and metabolic disease in adverse pregnancy outcomes such as small for gestational age and large for gestational age, and long term outcomes
- Effect of diet on normal development both in pregnancy and before pregnancy. Consider that it could exert a protective as well as negative influence on the offspring.
- The need for biomarkers that can be linked to epigenetic markers/changes.
Lactational Exposure and Risk
- The benefits of breast-feeding to both mother and child are widely recognized, but because of the lack of data most drug labels indicate that they should not be used during breast-feeding, or that the mother should not breastfeed when taking the drug.
- Lactational exposures are often ignored; to address this requires cultural shifts and communication
- With regard to exposure, milk assays should be performed routinely, and innovative approaches and/or incentives to obtain samples are needed. With regard to risk to the nursing infant, data are needed to understand “dose”, exposure, and potential adverse effects. Based on significant differences in physiology and milk composition, animal data are generally not considered very useful in evaluating secretion of medications and chemicals in milk.
- Finally, there is an emerging need to examine long term outcomes of lactational exposures.
Opioids
- Neonatal abstinence syndrome (NAS). As misuse and abuse of opioids is increasing in the US, NAS is expected to be an ongoing problem. Diagnosis and management, including development of tools and training of caregivers is a critical need.
- Opioid-induced neural tube defects (NTD). Research is needed to better understand the potential link of opioids to neural tube defects, including mechanistic studies to delineate whether neural tube defects related to opioids are due to maternal toxicity (e.g., respiratory depression) or direct effects on the embryo.
Emerging Technologies
- New technologies, including high throughput “omics”, microphysiological systems, modeling, computational and in silico tools are advancing rapidly. These technologies present tremendous opportunity to interrogate pathways/mechanisms of embryonic development and birth defects in powerful new ways.
- These new technologies generate big data. Database management, integration and access will continue to be a challenge in dealing with these data sets.
- The application of these technologies in developmental and birth defects research presents a need for skill sets (and the integration of skill sets) to tackle emerging issues not historically linked with our field, including statistics, informatics, computer science, engineering and math, in addition to developmental biology, epidemiology, chemistry and toxicology. This presents a critical need for training and recruitment in these disciplines and emphasizes the importance of multidisciplinary teams.
Open-ended
An additional break-out was held to brainstorm on other topics not previously identified.
- There is a critical need to integrate data sources, such as birth defect registries, clinical data, and large databases relating to exposures to better allow for cross-referencing information to identify emerging links or trends.
The level of engagement and outcomes of these discussions underscore the benefit of a multidisciplinary forum for identifying and addressing emerging needs in birth defects research. Successes have been realized when all interested and dedicated parties work together. Annual gatherings like the research needs workshop at the Teratology Society meeting can facilitate these discussions and the advancement of research towards understanding and prevention of birth defects. I hope you will join me for the next Teratology Society Annual Meeting in June 2019.
About the Author
Dana Shuey, PhD, DABT, is the Executive Director of Toxicology at Incyte, a biopharmaceutical company, and current President of the Teratology Society.
About the Teratology Society
To understand and prevent birth defects and disorders of developmental and reproductive origin, the Teratology Society promotes multi-disciplinary research and exchange of ideas; communicates information to health professionals, decision-makers, and the public; and provides education and training.
Scientists interested in or already involved in research related to topics mentioned in this blog are encouraged to join the Teratology Society and attend the 59th Annual Meeting June 22–26, 2019, the premier source for cutting-edge research and authoritative information related to birth defects and developmentally-mediated disorders. Teratology Society members include researchers specializing in cell and molecular biology, developmental biology and toxicology, reproduction and endocrinology, epidemiology, nutritional biochemistry, and genetics, as well as the clinical disciplines of prenatal medicine, pediatrics, obstetrics, neonatology, medical genetics, and teratogen risk counseling. In addition, the Teratology Society publishes the scientific journal, Birth Defects Research. Learn more at www.Teratology.org. Find the Teratology Society on LinkedIn, Facebook, and Twitter.
2018

By Janet Hardy, PhD, MSc, MPH
Teratology Society Member since 2005
This blog also appears on Medium.com and LinkedIn with additional photographs.
When a brave little girl sits in front of this white woman and reluctantly gives up an important possession….a tiny, dirty wad of tissue used to keep the drainage flowing from her ear onto her threadbare sweater….I am stripped to the core of my humanity and reminded just how profoundly privileged my life is. This woman is me, Janet Hardy, an epidemiologist whose specialties are medication safety in pregnancy, and maternal-child health research and programming in domestic and low resource regions. However, this past summer I stepped out of my research comfort zone. Every time I do this, it changes me.
Let me explain. I’ve worked with communities in Central America, India, and African countries, but over the summer I worked specifically in Philippi Township, Western Cape, South Africa on a project that involved young school-age children. It became known as “the ear project.” I was a member of a team of 26 that flew in from various parts of North America to work with a local team in this large township outside of Cape Town. Philippi is an informal settlement, where homes and businesses are constructed wherever space is available (i.e., metal shacks, attached or in very close proximity). “Informal” implies that the government doesn’t recognize it and, therefore, doesn’t provide basic services such as water/sewer, electricity, and garbage collection. There are over 200,000 people living in Philippi, of which over 90% are black African. Xhosa is the primary language.
We worked with school children in kindergarten through the third grade. Each day, the team traveled to work at a different school. Of note, the project took place in late July-August. This is winter-time in South Africa and so school was in session.
The Ear Project
Globally, acquired hearing loss is underdiagnosed, undertreated, and poorly understood. Ear infections are highly prevalent in South Africa and, in communities such as Philippi, the average child with a chronic ear infection may have an active infection with drainage for three years. Complications from these infections can include destruction of the internal ear structures that are needed for proper hearing. Brain abscesses can be the most deadly type of complication from these infections. Hearing loss, whether congenital or due to infections, can have a major negative impact on a child’s development, education, socialization, self-esteem, and future employment. Among the many daily concerns for families in Philippi, attending to this health problem is generally a low priority. There are approximately 12,000 students, or “learners,” in Philippi schools. The regional budget and small number of audiologists allow for school-based hearing screens of approximately 150 learners per year from the Philippi community in which we worked, largely based on referrals. It’s a woefully inadequate number relative to the magnitude of the problem.
The ear project began a few years ago with a physician colleague working in Durban, South Africa. The portion of the project that I was involved with last summer represented the largest scale-up effort attempted. We were essentially trying various processes to provide an efficient ‘pop-up’ school-based hearing/ear infection screening program. Our project scope included: classroom-based education on ear/hearing health, safety, and infection prevention (“nothing smaller than your elbow goes in your ear, even if you have a tickle”); and, for those children with a parent-signed consent form, we implemented a hearing screening program using new IOS-based technology and/or traditional audiometric technology, with or without an intervention/treatment or referral for more extensive care.
The project represented the collaboration of multiple entities working ahead of time to lay the groundwork as well as the pop-up field team (field team of approximately 40 people). The team collaborators included Amandla Development, Yale University (active faculty and alumni, who were predominantly clinical or allied health professionals), the University of Cape Town, the Red Cross War Memorial Children’s Hospital, the City of Cape Town Health Department, Western Cape Education Department, and the University of KwaZulu-Natal. Basic funding was provided through multiple sources. Additionally, in-kind and volunteer service was provided. As an alum, I provided my time as volunteer service through the Yale Alumni Service Corps.
What We Found
Our team screened over 1,530 children, an accomplishment that we were proud of. Approximately 10% of children screened in our program had pathology. The final analysis is not yet available. However, I worked with a high proportion of little children who failed their hearing tests, many children with burst eardrums and drainage due to infection…and many children with one or more foreign objects that had evidently enjoyed a long residence in their host’s ear. Ten children required immediate surgery. Six children were found to have cholesteatoma, an abnormal growth in the ear. Cholesteatoma may occur as a birth defect, but it more commonly arises following repeated middle ear infections. Untreated, it can result in hearing loss, brain abscess, meningitis, and, rarely, it can be fatal. Published occurrence estimates for cholesteatoma are few although there seems to be some consensus around 1 in 16,000. Suffice it to say, our finding 6 cases in 1,530 screened children was higher than expected and most of our North American team clinicians had never seen a case in their careers to date.
Life-Long Impact
Projects in global low resource regions are the highlight of my year, regardless of whether they are funded projects or volunteer efforts. Why do I do it? Because each time I get involved in such a project, I learn about myself; because I hope that I can make at least one life a little easier; because I learn how to be a better research scientist by understanding how others live and the struggles they face. I am reminded that the education I received was a gift and that it's mine to pass on as best I can.
I would urge scientists in any career stage to periodically step outside of your comfort zone: To get involved with fieldwork in a community that is unlike your own, domestically or globally, and to learn, deeply, what it is like to interact with communities who can benefit the most from your skills. You may surprise yourself at the breadth of skills you’ve acquired during your education and career-they are indeed greater than the specific degree you graduated with and the specialty of your research. You’ll return home and you’ll never look at your data as just numbers and covariates again, and you’ll think about the missing data. Because now, the numbers and covariates have faces and voices and the secrets you learned from these beautiful communities are also carried by those in the USA and elsewhere in the western world. Not only might it make you think more deeply about your research, but about your humanity.
About the Author
Janet Hardy, PhD, MSc, MPH is Assistant Professor of Epidemiology/Biostatistics (affiliate) at U. South Florida Dept. Epidemiology and Managing Principal of ECC Population Health Group which provides expertise-driven research & programmatic consulting to nonprofit, for profit & legal organizations in: 1) maternal-child health in domestic/low-resource settings; and 2) pharmacoepidemiology, women & child health. Dr. Hardy has been a Teratology Society member since 2005 and has served on the Education, Program and Science Committees.
About the Teratology Society
To understand and prevent birth defects and disorders of developmental and reproductive origin, the Teratology Society promotes multi-disciplinary research and exchange of ideas; communicates information to health professionals, decision-makers, and the public; and provides education and training.
Scientists interested in or already involved in research related to topics mentioned in this blog are encouraged to join the Teratology Society and attend the 59th Annual Meeting June 22–26, 2019, the premier source for cutting-edge research and authoritative information related to birth defects and developmentally-mediated disorders. Teratology Society members include those specializing in cell and molecular biology, developmental biology and toxicology, reproduction and endocrinology, epidemiology, nutritional biochemistry, and genetics, as well as the clinical disciplines of prenatal medicine, pediatrics, obstetrics, neonatology, medical genetics, and teratogen risk counseling. In addition, the Teratology Society publishes the scientific journal, Birth Defects Research. Learn more at www.Teratology.org. Find the Teratology Society on LinkedIn, Facebook, and Twitter.
Published June 2018

By Dana L. Shuey, PhD, DABT
I’m sometimes asked why I do what I do. Why am I involved with birth defects research? Why am I passionately part of a Society that works to better understand birth defects and prevent them? My answer—it literally can affect every person on Earth, and the impact on individuals is lifelong. Birth defects and reproductive and developmentally mediated disorders remain a major global public health concern. Recent events make that clear. The Zika epidemic and the ongoing opioid crisis underscore the importance of understanding potential risks of maternal exposures and environmental factors to the developing fetus.
Critical to the understanding of birth defects are contributions from many diverse research areas, from basic developmental biology, genetics, cellular and molecular biology, toxicology, computational and systems biology, clinical practice and epidemiology, and risk assessment. This diverse, multidisciplinary and translational approach to the understanding and ultimate prevention of adverse developmental outcomes will be showcased at the upcoming 58th Annual Meeting of the Teratology Society June 23–27 in Clearwater Beach, Florida. The meeting provides an important forum for the presentation of new research, exchange of ideas, and fostering collaboration among scientists across disciplines. Sessions at the meeting will present emerging research on the effects of maternal and environmental factors on offspring, including infectious diseases, botanical supplements, and air pollution, as well as evaluating the impact of a variety of public health approaches to education and prevention of birth defects, and challenges in the development of new therapeutics for the treatment of neonates and rare childhood diseases.
This multidisciplinary approach is important to the fight against direct threats to future generations, like the Zika virus. Just ask Celeste Philip, MD, MPH, our highly anticipated Keynote Speaker and current Surgeon General and Secretary of the Florida Department of Health. “We are still working toward solutions like vaccine development, point-of-care testing, and proven vector control methods to ensure that Zika never poses as much of a threat as it did in 2016,” she said. “Mosquito-borne illness will always be a threat in Florida, but we are certainly not unique in that regard, so it is especially crucial for us to convey the importance of prevention to the public and our partners on both a local and global scale,” she added.
Additional areas of concern affecting communities far and wide include:
Progress in the Understanding of Autism
Autism Spectrum Disorders (ASDs) have been estimated to occur in as many as 1 in 59 children, and often occurs with other developmental, psychiatric, neurologic, chromosomal, and genetic diagnoses. Environmental and genetic factors leading to ASDs are poorly understood. Progress in understanding the embryological origins of ASD will be presented.
The Impact of Opiate Use on Pregnancy and Child Development
The use of opioid drugs is a critical and growing public health crisis, with a high potential risk in pregnancy. The impact of this crisis on pregnancy and the developing fetus and emerging research on the underlying neurodevelopmental effects of prenatal opioid exposure from animal and human research will be highlighted.
Application of New Technologies in the Understanding of Birth Defects
Research methods, new models and technologies are emerging quickly, allowing researchers to explore new questions related to the fundamental understanding of mechanisms causing birth defects. Several of these new technologies will be highlighted during the meeting. The development and application of organotypic culture models using human cells have provided powerful new tools to investigate pathways of normal and abnormal development. Genomic sequencing has revolutionized our understanding of the causes of birth defects, and the current status of genome-wide sequencing as a diagnostic test for genetic causes of birth defects will be discussed. Epigenetic regulation of gene expression, including DNA methylation, posttranslational histone modification, and non-coding RNAs, is critical to the development of all organ systems and has become increasingly recognized as a target for environmental influences on developmental outcomes.
About the Author
Dana Shuey, PhD, DABT, is the Executive Director of Toxicology at Incyte, a biopharmaceutical company, and current Vice President of the Teratology Society.
About the Teratology Society
To understand and prevent birth defects and disorders of developmental and reproductive origin, the Teratology Society promotes multi-disciplinary research and exchange of ideas; communicates information to health professionals, decision-makers, and the public; and provides education and training.
Scientists interested in or already involved in research related to topics mentioned in this blog are encouraged to join the Teratology Society and the 58th Annual Meeting June 23–27, 2018, the premier source for cutting-edge research and authoritative information related to birth defects and developmentally-mediated disorders. Teratology Society members include those specializing in cell and molecular biology, developmental biology and toxicology, reproduction and endocrinology, epidemiology, nutritional biochemistry, and genetics, as well as the clinical disciplines of prenatal medicine, pediatrics, obstetrics, neonatology, medical genetics, and teratogen risk counseling. In addition, the Teratology Society publishes the scientific journal, Birth Defects Research. Learn more at www.Teratology.org. Find the Teratology Society on LinkedIn, Facebook, and Twitter.
This blog is also available on LinkedIn here. All past Birth Defects Insights blog may be found here.
Published January 2019

By Asher Ornoy, MD
Also see this blog on LinkedIn.
It’s a drug group being used more than any other group in pregnancy. They’re drugs often discussed in the popular media, particularly when depression, attention deficit hyperactivity disorder (ADHD) and autism spectrum disorder (ASD) are discussed. It’s a group being used by as many as 3–5% of pregnant women and, theoretically, might have various effects, possibly even harmful ones. I’m talking about selective serotonin reuptake inhibitors (SSRIs) and selective serotonin norepinephrine reuptake inhibitors (SNRIs), relatively large groups of drugs that increase the levels of serotonin (and norepinephrine) in the serotonergic (and noradrenergic) synapses. These drugs are used for the treatment of depression, anxiety and a variety of other mood disorders, but the confusion about their effects on a developing fetus during pregnancy is palpable. Additionally, while there is agreement that SSRIs and SNRIs do not increase the rate of congenital malformations in general, there might be some increase in the rate of certain types of birth defects and various transient negative effects on postnatal adaptation.
The Rise of ASD and ADHD
Due to the alarming increase in the last two decades in rate of ASD as well as other neurodevelopmental problems including ADHD, there is increasing interest in the possible role of prenatal factors in the etiology of these disorders, especially ASD. As a result, many studies evaluating the possible relation between prenatal medications and ASD are being published.
The most solid evidence that an association between prenatal events and ASD may exist is demonstrated with prenatal exposure to the antiepileptic drug valproic acid. Several studies show a 5–10 times increase of ASD among children prenatally exposed to valproic acid. Valproic acid has induced ASD-like symptoms in experimental animal models. In addition, similar associations were reported with several other drugs taken in pregnancy for treatment of depression, including SSRIs. Several case control studies, as well as population registry studies indeed showed about a 50% increase in the rate of ASD following prenatal exposure to SSRIs. Now before you say, “We have an answer!” it’s important to note that several large population-based studies fail to show any association of prenatal SSRI exposure and ASD.
The Confusion Continues
Even meta-analyses and literature reviews show opposing data, and the denial or acceptance of an association depends on the exclusion and inclusion criteria in these meta-analyses. Similarly, some studies reported on a possible association of SSRIs with an increased rate of ADHD. However, other studies showed an association of ASD and ADHD with maternal depression, not related to the SSRIs. In a recent meta-analysis, an association was found between preconception use of SSRIs and ASD but not with its use during pregnancy, clearly demonstrating that the association seems to be between maternal depression and ASD Moreover, depression seems to be a genetic and environmental disorder. ASD similarly has a genetic and environmental origin. Both disorders might share common genes, which could explain the association between depression and ASD.
As far as other possible correlations are concerned, our own cohort studies showed a slightly higher rate of cardiac anomalies in the offspring of women treated with fluoxetine and paroxetine, but no other pregnancy complications, except for difficulties in perinatal adaptation- i.e., adaptation of the newborn infant to extrauterine life. Moreover, some newborn infants also have withdrawal symptoms that do not seem to have long-term consequences.
Turning Insufficient into Sufficient Once and for All: Where the Science Needs to Improve
In spite of the disagreement in the medical literature, it can be summarized that there is insufficient evidence for a definite association of prenatal SSRIs and ASD or ADHD, as most studies fail to control for maternal disease, for postnatal effects of a non-optimal home environment and especially for genetic factors that might be common to various psychiatric disorders. It seems clear, when reading most robust literature that most of the large population-based studies on the possible effects of SSRIs in pregnancy are based on prescriptions of these drugs during pregnancy, and some studies are based on only one prescription. However, having a prescribed drug does not mean that the drug is taken. Hence, this is a source of potential bias. There are studies showing that many of the women that are prescribed drugs do not take them, especially when they plan pregnancy. Moreover, in many studies there are no appropriate control groups of untreated women with depression.
The literature on neurodevelopmental outcomes generally does not show significant neurodevelopmental damage induced by SSRIs, but the data are mainly on young children, and there seems to be no data on possible effects at adolescence. This is especially important since it was shown that maternal depression may be associated with behavioral changes at adolescence.
More on the Role of Maternal Depression during Pregnancy
Maternal depression (and anxiety, as they often go together) especially if untreated, may have a profound impact on child development at early life, at school age and at adolescence, thus influencing life-long behavior. In a recent Canadian study, half of a large population of mothers with depression and anxiety disorders were being treated with antidepressants. Their children were examined in kindergarten at the age of 5–6 years. The investigators found an inverse relationship between maternal depression and a child's achievement on an early childhood assessment test. Children born to mothers with depression/anxiety had more problems in communication skills, emotional maturity, language and social competence.
Maternal depression during pregnancy and recurrence of depressive episodes had a higher negative influence on development compared to postnatal (postpartum) depression. These negative influences are more severe in children of parents of lower socio-economic class. These effects were noticed in infants as well as in school aged children. Adolescents raised during childhood by depressed mothers were more likely to use illicit substances, to engage in risky and/or violent acts and have delinquent behavior also disobeying laws. All these problems were in direct relation with the severity of maternal symptoms. As a result, it seems that appropriate treatment for depression/anxiety during pregnancy (as well as postpartum) is important, especially if the symptoms are severe. Pharmacological treatment could be offered if the other means of treatment are inadequate. In favor of pharmacological treatment is also the fact that possible negative effects on the fetus of antidepressants in pregnancy are low, if any.
Getting Concrete Answers about Antidepressant Safety in Pregnancy
Often in studies of medications, it is difficult to disentangle the effects of the medication from the effects of the disease that the medication is being used to treat. This challenge is otherwise known as “confounding by indication.” One of the most important issues in epidemiological studies evaluating the safety of antidepressant use during pregnancy is ensuring that confounding factors are controlled for. Moreover, when comparing the outcome of treated and untreated patients with similar psychiatric disorders, one must remember that the women who are treated with SSRIs (or with other antidepressants) generally have a more severe disease compared to those that are not treated. In addition, there are genetic differences in the way people metabolize SSRIs, which should be considered. This is mainly due to differences in the liver cytochrome P 450 enzymes (CYP enzymes) which are the major metabolic pathways for SSRIs and SNRIs. Similarly, there are differences in the metabolism of the various drugs in these groups. Hence, the difficulties in getting precise answers are obvious. It seems that only prospective follow up cohort studies that will accurately assess the severity of maternal depression, long-term children’s neurodevelopmental outcome including their mental status as well as proper genetic studies might give more definitive answers.
Studies have shown that many genes are associated with depression, ASD and ADHD. However, the way they interact with each other and with the environment is still largely speculative. Only after we understand these interactions might we find the way for their prevention. How far we still have to go is merely speculative.
About the Author
Raised by parents who had survived World War II concentration camps, Asher Ornoy, MD, Professor of Embryology, Teratology and Medical Neurobiology at the Hebrew University Hadassah Medical School, had examples of hard work and perseverance throughout his childhood, inspiring him to work hard at helping others the best way he believed he could–first as a doctor, and in addition as a researcher with a particular interest in prenatal and postnatal development of children. He received his medical degree from Hebrew University Hadassah Medical School in Jerusalem, Israel and Pediatric training at Hadassah University Hospital. He had additional training in clinical teratology and in child development in the Children's hospital, Cincinnati University Medical School, and training in pediatric neurology at the Jefferson Medical College in Philadelphia, PA. He has been a Teratology Society member since 1978. He published about 320 papers in peer reviewed journals in the fields of teratology and child development. Most recently, he was a co-author, along with Kembra Howdeshell, PhD, of a review of the science on the safety of antidepressant use during pregnancy and lactation in the Teratology Society’s Journal, Birth Defects Research: Depression and its Treatments during Pregnancy. It can be read here: http://onlinelibrary.wiley.com/doi/10.1002/bdr2.v109.12/issuetoc
About the Teratology Society
Scientists interested or are already involved in research related to topics mentioned in this blog are encouraged to join the Teratology Society and the 58th Annual Meeting June 23–27, 2018, the premier source for cutting-edge research and authoritative information related to birth defects and developmentally-mediated disorders. Teratology Society members include those specializing in cell and molecular biology, developmental biology and toxicology, reproduction and endocrinology, epidemiology, nutritional biochemistry, and genetics, as well as the clinical disciplines of prenatal medicine, pediatrics, obstetrics, neonatology, medical genetics, and teratogen risk counseling. In addition, the Teratology Society publishes the scientific journal, Birth Defects Research. Learn more at www.Teratology.org. Find the Teratology Society on LinkedIn, Facebook, and Twitter.
Suggested Reading:
Ornoy A. Neurobehavioral risks of SSRIs in pregnancy: comparing human and animal data. Reproductive Toxicology 72:191–200, 2017.
Ornoy A, Koren G. Selective Serotonin Reuptake Inhibitors during pregnancy: do we have now more definite answers related to prenatal exposure. Birth Defects Research Part C. 109(12):898–908, 2017.
Comaskey B et al. Maternal depression and anxiety disorders (MDAD) and child development: a Manitoba population-based study. Plos One 12(5): e0177065, 2017. Available at: https://doi.org/10.1371/journal.pone.0177065
Wickham ME et al. Maternal depressive symptoms during childhood and risky adolescent health behaviors. Pediatrics. 135(1):59–67, 2015.
Howdeshell, K, Ornoy, A. Depression and its treatment during pregnancy: Overview and Highlights" Birth Defects Research Part C. 109(12):877–878, 2017
2017
Published November 2017

By John W. Kille, PhD, DABT
See and share this blog on LinkedIn.
It’s really no surprise why those little packets brightly colored and available at a diner near you have risen in popularity just as quickly as it takes to tear them open and dump in our coffees and iced teas. Most commonly used artificial sweeteners are high intensity sweeteners, which means that their sweetness intensity is many times greater than that of sugar (from 100 up to 20,000 times as sweet). But as a toxicologist with decades of experience evaluating data related to the safety of food additives during pregnancy, I can’t help but ask the question–Is our sweet obsession souring the health of fetal development? It’s an important question to answer as some consumer reports indicate the consumption of artificial sweeteners will continue to rise over the next ten years.
The Bottom Line:
Most pregnant women can safely ingest any artificial sweeteners approved by the international regulatory agencies and health authorities. The three main artificial sweeteners are: saccharin (e.g. Sweet’NLow), aspartame (e.g. NutraSweet and Equal), and sucralose (e.g. Splenda), although there are others, described below. These sugar substitutes are used in many different types of foods and drinks, but add little or no nutritional value. Women who have phenylketonuria are a special exception to the safety of the artificial sweetener, aspartame, and may need to avoid it during their pregnancies. Unrefined Stevia, a natural sweetener that is used as a sugar substitute, has not been approved for pregnancy, but its extract Rabaudioside-A, a purified glycoside of the Stevia plant, is considered safe for use during pregnancy under the FDA’s GRAS (Generally Recognized As Safe) classification for food additives. These differences are explained in greater detail below.
Currently, there are six high-intensity sweeteners that have been approved as safe food additives by the FDA. These are (sweetness intensity X that of sugar): saccharin (100-700X), aspartame (200X), acesulfame potassium (Ace-K, 200X), sucralose (600X), neotame (7000-13,000X), and advantame (20,000X). There are other sweeteners that are not high-intensity, but are non-nutritive. These are plant-derived and are considered safe by GRAS affirmation (see below).
But…(Yes, there is a “but”)…There are a Few Basic Facts and Cautions to Keep in Mind:
In general, a new food additive such as an artificial sweetener must go through rigorous testing in the laboratory and in animals, including pregnant animals, and clearly demonstrate safety before the FDA or any international regulatory body will allow its use in foods. These, for the most part, have undergone very thorough testing prior to approval, and then toxicology specialists in the regulatory agencies critically analyze the data as it applies to human safety before they can be used in foods and beverages. The fact that most artificial sweeteners are high intensity sweeteners minimizes the amount that is necessary to provide the sweetness equivalent of a teaspoon of sugar. Therefore, your body is exposed to much less of the high intensity sweetener than it would be to sugar for the same level of sweetness. This also reduces the potential for adverse effects. In addition, most are non-nutritive, which means that they provide no calories or so few calories that they are considered non-caloric. This is the property that makes them attractive as adjuncts to a reduced-calorie diet. The other side of that coin, however, is that “non-nutritive” means there is no nutritional benefit obtained by the mom-to-be or her baby when consuming these sweeteners.
One further point, non-nutritive sweeteners are widely divergent in their chemical structure, which means they are not likely to undergo the same chemical transformations in your body, or be metabolized the same way. So, any claims you read of biological or chemical actions attributed to non-nutritive or high-intensity sweeteners as a class, other than eliciting the sensation of sweet taste, are likely to be inaccurate or at least an over-simplification of complex biochemical responses. Just because one non-nutritive sweetener may be associated with a particular response, safety or otherwise, it is not correct to assume that all non-nutritive sweeteners do the same thing (as is often claimed, even by scientific researchers) unless they are individually tested under the same conditions and shown to produce the same results.
An excellent overview of high intensity sweeteners and their safety is available on the FDA website.
A Closer Look at the Three Main Artificial Sweeteners in Foods Today:
Saccharin
The oldest artificial sweetener is saccharin. Saccharin has been consumed for a long time and has not been shown to cause birth defects. It was discovered in 1879 and for a century or more, it was the only non-carbohydrate sweetener available to diabetics or diet-conscious consumers that could replace sugar until aspartame was approved in 1980. This was followed somewhat later by other sweeteners such as sucralose, which was approved in 1998. Since saccharin is not a sugar, diabetics can use it without a rise in blood glucose. Because of this, and despite its metallic off-taste, its consumption and popularity steadily increased over the years, even to the extent that some preferred the taste over sugar (e.g. Tab cola with lemon). The discovery of saccharin preceded the organization of the FDA, so the rigorous safety testing that other high intensity sweeteners have undergone was not required for saccharin when it was introduced. Several safety studies have been conducted over the intervening years, most of which confirmed its safety. However, in the late 1960s lifetime animal studies using a combination of saccharin and another sweetener, cyclamate, produced tumors in the bladders of rats. Saccharin and cyclamate were placed on a U. S. list of likely human carcinogens, and FDA threatened to remove saccharin from foods. But, due to the public outcry stemming from the potential removal of the only approved sugar substitute in the US at that time, Congress overruled the FDA (the only time this has occurred) and kept saccharin on the market. Later scientific studies, however, showed that the bladder tumors occurred only in male rats and were due to a combination of events. These included the production of a urinary protein that was the responsible cancer-instigating agent. That protein, however, was only formed in male rats, not in humans. Under certain conditions of urinary pH and testosterone the protein crystallized in the rat bladder causing irritation that ultimately led to the tumors. These studies, as well as epidemiologic studies in humans, showed that there was no human cancer risk, and saccharin was later removed from the list of possible cancer-causing agents by the National Institute of Environmental Health Sciences. The National Cancer Institute reported that there is no scientific evidence that any of the artificial sweeteners approved for use in the United States cause cancer. Nevertheless, while saccharin gained a scientific reprieve on its safety, cyclamate (the other sweetener in the rat bladder study) remains unapproved in the US as a potential carcinogen. It is an approved sweetener in Canada, but then only if sold directly to the consumer, not as an ingredient in food products. Further, it must be identified as such on a label, and the label must also contain a statement indicating cyclamate should only be used in foods under the advice of a physician.
Aspartame
Aspartame is not a sugar. It is a peptide composed of two amino acids (the building blocks of proteins), phenylalanine and aspartic acid, which together activate the sweetness receptors. Because it is not a carbohydrate like sugar diabetics can consume it without raising their blood glucose levels. It has an extensive safety database (approximately 200 studies) demonstrating its safety over a wide range of food uses and conditions. These studies, including investigations on fertility and embryo/fetal development, show no evidence that it causes birth defects except perhaps in pregnancies affected by an inherited maternal genetic defect (see below). Although some members of the public and a few clinicians have raised concerns about its safety, most physicians and scientists, including FDA experts, as well as toxicologists in all international food regulatory review panels have determined that it is safe for use during pregnancy.
The one very important exception is in pregnant women who have a rare hereditary genetic disease called phenylketonuria, or PKU. These moms-to-be may need to avoid the phenylalanine contained in aspartame because they lack an enzyme that allows them to break down the phenylalanine into safe chemical components. If they don't limit phenylalanine intake from all sources during pregnancy, excess phenylalanine can build up in their body, and in their fetus, and can cause brain damage and cognitive impairment in their baby even if the baby does not carry the defective PKU gene. PKU occurs in approximately 1 out of 10,000 to 15,000 babies in the U.S. with higher rates of occurrence in whites and Native Americans than in blacks, Hispanics and Asians. Fortunately, PKU is readily identified in babies as part of routine newborn screening. When PKU is detected, a special medical diet designed by a neonatal nutritionist can prevent the negative effects of PKU if started early enough and followed throughout childhood and adolescence. The clinical manifestations of PKU may be variable and some individuals with PKU are able to be less vigilant about their diet as they enter adulthood. However, research has shown that a return to a strict limiting of phenylalanine intake is necessary during pregnancy to protect the developing fetus. Fortunately, thanks to FDA-mandated labeling of products containing aspartame, avoiding phenylalanine is relatively easy for the few expectant moms with PKU.
SucraloseSucralose is a non-nutritive sweetener that closely resembles table sugar (sucrose) in both taste and chemical structure. The difference from sugar is that it has been chemically transformed and contains three chlorine atoms substituted in very specific locations in the molecule. These chlorine atoms do not come off and pose no adverse risk. Their specific locations in the molecule are responsible for the 600-fold increase in sweetness over sugar. But, more importantly, because of these changes in the molecule the body does not recognize sucralose as a sugar, so it is not able to digest and metabolize it as a source of calories. Furthermore, because the body doesn’t recognize it as a sugar, diabetics can consume it safely without affecting their blood glucose, insulin or hemoglobin A1C. Bacteria in the mouth likewise cannot metabolize sucralose, and that means that dental plaque bacteria cannot use it to produce the acid that causes tooth decay. The body does not readily absorb it, and 85% is eliminated in the feces unchanged. The small amount that is absorbed is processed safely by the body and eliminated in the urine. It does not cross the placenta nor enter the fetus. Sucralose has undergone an extensive series of safety studies (over 110), including those in pregnant animals to determine whether there are any potential adverse effects on sperm metabolism, fertility, embryo/fetal development including birth defects, or on any other aspect of the reproductive process. These studies have shown no adverse effects on fertility, pregnancy, or the normal development of offspring.
In general, the vast majority of people have no problems consuming artificial sweeteners. But there are reports of some who may be sensitive to one or another sweetener and may develop unexpected responses after consuming it (e.g. headache, GI distress). If you do experience some disturbance after drinking a diet drink or eating food that contains one of these sweeteners there is no known increased risk to your baby, but for your own comfort you may want to try products that use a different sweetener.
Other types of sweetener substitutes
There are other types of sweetener substitutes that are usually not high intensity sweeteners. These are plant-derived, for example, from sources such as the Stevia plant. These generally have not undergone the same rigorous safety testing as is required for a new food additive such as the high intensity sweeteners because they have some history over many years of being used as a food without adverse consequences. Nevertheless, in order to affirm safety for a quality product the manufacturer usually undertakes what is known as a GRAS (Generally Recognized As Safe) Notice to FDA supporting its safety as a food. While GRAS evaluations are voluntary, in order to be acceptable to FDA these are quite detailed in their assessment of safety and must be compiled by scientists qualified by training and experience in evaluating safety data in order for the conclusions of safety to be considered valid. If submitted to the agency (this is not required), FDA may choose to review it and challenge the assessment. If FDA does not challenge it, the assessment of safety is allowed to stand and is considered a tacit “approval,” but one that may be challenged at any time the Agency finds it necessary, e.g. due to new information.
Rabaudioside-A, for example, is a purified glycoside of the Stevia plant (Stevia rebaudianaBertoni ) that produces non-nutritive sweetness and is an ingredient in several foods. This has undergone a GRAS review that has not been challenged by FDA and is, therefore, accepted as safe for the general public including pregnant moms.
Note, however, that whole leaf Stevia and crude Stevia extracts are not approved by regulators as safe sweeteners because there are reports in the scientific literature of adverse health effects that raise concerns, including cardiovascular and reproductive effects that FDA considers serious enough to withhold approval and warn against their consumption. One critical take-home message here is that just because something is natural, it does not necessarily mean that it is safe, or any safer, than a manufactured or processed product. Many natural products have potent components that can produce undesirable health consequences. Where your health and the health of your baby are concerned, a product must be established safe by qualified experts and not assumed to be safe just because it is “natural”.
Digesting the Facts
Based strictly on high quality scientific evidence, FDA and other international regulatory authorities have concluded that the approved high-intensity sweeteners are safe for the general population, including pregnant women and their babies, under the conditions of use as a food additive. There is no evidence of birth defects or any other adverse pregnancy effect associated with these sweeteners.
But… (There’s that “but” again.)
One health consideration for pregnant women who use artificial sweeteners is that they may be missing out on more nutritious foods and beverages. For example, if you drink a lot of diet soda, you may be getting the fluids that a healthy diet recommends; however, you won’t be getting the nutrients found in milk or juice, which are beneficial to you and your developing baby. So, if you are pregnant, or you work with pregnant patients, be sure (to encourage them) to eat a balanced diet and drink a variety of healthy beverages.
About the Author
John W. Kille, PhD, is the founder and principal of the consulting firm J. W. Kille Associates, which provides services in toxicology, regulatory affairs, risk assessment, and other areas related to the safety evaluation and development of food additives, pharmaceuticals, and medical devices. He has over 35 years' experience conducting experiments and evaluating data related to the safety of food additives and pharmaceuticals, specializing in the area of effects on pregnancy and embryo/fetal development. He served as the principle reproductive toxicologist on the development of sucralose for McNeil Specialty Products.
Dr. Kille received his BA from Lafayette College, his MS from Villanova University in biology (embryology), and his PhD from the University of Virginia in developmental biology. He attained board certification in toxicology from the American Board of Toxicology in 1985.He is a Teratology Society member and is currently Past Chair of the Constitution and Bylaws Committee.
About the Teratology Society
Scientists interested or are already involved in research related to topics mentioned in this blog are encouraged to join the Teratology Society and the 58th Annual Meeting June 23–27, 2018, the premier source for cutting-edge research and authoritative information related to birth defects and developmentally-mediated disorders. Teratology Society members include those specializing in cell and molecular biology, developmental biology and toxicology, reproduction and endocrinology, epidemiology, nutritional biochemistry, and genetics, as well as the clinical disciplines of prenatal medicine, pediatrics, obstetrics, neonatology, medical genetics, and teratogen risk counseling. In addition, the Teratology Society publishes the scientific journal, Birth Defects Research. Learn more at www.Teratology.org. Find the Teratology Society on LinkedIn, Facebook, and Twitter.
References:
Saccharin–development of bladder tumors, relevance to humans
Incidence and treatment of PKU in the US
Sucralose, no effects on reproduction
Sucralose, assessment of teratogenic potential (birth defects)
By Alan M. Hoberman, PhD, DABT, ATS
“US Women Increasingly Use Pot during Pregnancy,” it’s a headline I’m staring at right now as I write this. It’s research being published in journals like JAMA. It’s a potential risk to future babies everywhere—like in Colorado where recreational marijuana use has reportedly increased among the pregnant population. Is getting high in the Mile High City worth any potential risks to the fetus?
Enter teratologists.
Teratologists study exposures during pregnancy that could potentially affect a developing fetus. So what better place to talk about the latest research surrounding marijuana use in pregnancy than in Denver? Marijuana use among pregnant women has risen by as much as 62% according to some reports examining data over a period of time during which several states, including Colorado, have legalized marijuana. In June, hundreds of the world’s leading researchers will convene in Denver at the Teratology Society's 57th Annual Meeting and present research about marijuana exposure in pregnancy, but also reveal research being published right now on Zika virus, e-cigarettes, cocaine and much more.
What mothers eat, drink and are exposed to in the environment, may have a long and lasting impact on their children. Take alcohol, for example. We have known about many of the adverse consequences of prenatal alcohol exposure for years, and yet we continue to learn more and more about its effects on a developing fetus. Did you know prenatal alcohol exposure affects each woman differently and, as a result, each developing baby differently? There’s no “one-size-fits-all” when it comes to a “safe” amount during pregnancy. Mom’s metabolism, genetics and so many other factors can affect outcomes. That’s just a small example of some of the research presented during past annual Teratology Society meetings by world-renowned investigators, such as pediatrician Kenneth Lyons Jones, MD, a pioneer in the area of Fetal Alcohol Syndrome.
From alcohol to e-cigs.
Today, we have e-cigarette exposure (either primary or secondary) in the illicit drug use category that has the potential to affect a fetus. It’s a relatively new risk to the pregnant woman, and the complication of legal and illicit drug and alcohol use only further complicates our ability to ensure that our next generation is healthy. E-cigarette use may be another critical prenatal co-exposure to consider just as much as cannabis in Colorado and other states. To be able to get information on how these newer types of co-exposures affect developing babies, as well as the latest information on alcohol use during pregnancy, is of great interest to me not only as a scientist, but also as a citizen. Colorado’s chief medical officer Larry Wolk, MD will put it all into context in his Keynote Address talking about the impact of marijuana legalization on children and families thus far.
Don’t forget genetics.
This year I am particularly looking forward to hearing about CRISPR/Cas-9 genome editing from Jacob Corn, PhD, UC Berkeley. This next generation of genome editing of the animal and human genome opens the possibility of improving the lives of persons with many of the human genetic diseases that Teratology Society members have characterized, studied and presented over the years at Annual Meetings.
We are on the brink of cutting-edge, exciting breakthroughs in genetics, developmental biology, epidemiology, reproduction, prenatal medicine and many other disciplines. The annual Teratology Society meeting allows the issues that cross these disciplines to be discussed, leading to new areas of research and improving treatment options for the fetus and infant, highlighting the importance of birth defects research and, in turn, improving societal health. I hope my fellow colleagues, students and postdoctoral researchers and health care professionals will join me in witnessing these exciting breakthroughs firsthand in Denver. #TS2017 #IamTeratology
About the Author
 Dr. Alan M. Hoberman is a 40-year veteran in toxicology who has specialized in reproductive and developmental toxicology for over 34 years. Currently, he is responsible for designing, supervising and evaluating reproductive, developmental, and juvenile toxicity studies throughout Charles River Laboratories. He is a Diplomate of the American Board of Toxicology, a fellow of the Academy of Toxicological Sciences and is the current Vice President of the Teratology Society. Dr. Hoberman has authored over 85 publications and presented 200 abstracts and lectures in the fields of reproductive and developmental toxicology, neurotoxicology, inhalation toxicology, photobiology and regulatory toxicology.
Dr. Alan M. Hoberman is a 40-year veteran in toxicology who has specialized in reproductive and developmental toxicology for over 34 years. Currently, he is responsible for designing, supervising and evaluating reproductive, developmental, and juvenile toxicity studies throughout Charles River Laboratories. He is a Diplomate of the American Board of Toxicology, a fellow of the Academy of Toxicological Sciences and is the current Vice President of the Teratology Society. Dr. Hoberman has authored over 85 publications and presented 200 abstracts and lectures in the fields of reproductive and developmental toxicology, neurotoxicology, inhalation toxicology, photobiology and regulatory toxicology.
About the Teratology Society
Scientists interested or are already involved in research related to topics mentioned in this blog are encouraged to join the Teratology Society and the 57th Annual Meeting June 24–28, 2017, the premier source for cutting-edge research and authoritative information related to birth defects and developmentally-mediated disorders. Teratology Society members include those specializing in cell and molecular biology, developmental biology and toxicology, reproduction and endocrinology, epidemiology, nutritional biochemistry, and genetics, as well as the clinical disciplines of prenatal medicine, pediatrics, obstetrics, neonatology, medical genetics, and teratogen risk counseling. In addition, the Teratology Society publishes the scientific journal, Birth Defects Research. Learn more at www.Teratology.org. Find the Teratology Society on LinkedIn, Facebook, and Twitter.
Published January 2017
By Sonja A. Rasmussen, MD, MS
President, Teratology Society
What a difference a year makes. In December 2015, as incoming Teratology Society president and chair of the 2016 annual meeting program committee, I was looking for an issue for our “Hot Topics” session. Each year, we save time at our annual meeting for an emerging topic and time was getting short. I remembered an email the Teratology Society office had received in late October: “. . .we are concerned about the introduction of a new arbovirus in the continent, in particular chikungunya and Zika viruses, and would like to provide tools to the countries to study and identify potential congenital arboviral infections, including their possible teratogenicity.” At that time, I worked to connect the person with our CDC experts in birth defects surveillance and in arboviral diseases. I recalled seeing more on this topic in the news—reports about an outbreak of Zika virus that had been linked to an increased number of babies born with microcephaly in Brazil. I proposed this topic to the Teratology Society Science Committee. Initially, there was some skepticism, but overall, committee members supported the topic. One member commented, “This looks to be a fascinating topic if the findings are real. (Remember the anencephaly outbreak in Brownsville, TX that was thought to be due to pollution or due to a high corn diet with resulting low folate?)” After some discussion, we decided to go with the topic—but to leave the title vague, in case by June, it was clear that this was a red herring. We called the session “Exploring the Link between Zika Virus and Adverse Pregnancy and Birth Outcomes”.
Of course, a year later, we now know a lot more about Zika and the problems it can cause during pregnancy (see this recent summary.) Our Teratology Society Annual Meeting and Zika session in June 2016 were successful, with presentations from colleagues from Brazil and CDC sharing the latest information on the outbreak. Now Zika has spread beyond Brazil—to 49 other countries in the Americas, including the United States. Using criteria set by one of the Teratology Society founders, Dr. Tom Shepard, Zika virus was determined to be a cause of microcephaly and other serious brain defects. A recognizable pattern of defects, called congenital Zika syndrome, has been described in infants born to mothers infected with Zika virus during pregnancy. We are beginning to understand the pathogenesis of Zika—that it is neurotropic with a special affinity for neural progenitor cells. Animal models for Zika and microcephaly have been developed, and work on a Zika vaccine is well underway.
Still, many questions remain.
What other health and developmental issues can we attribute to Zika infection during pregnancy? How do other factors such as previous infections, a woman’s metabolism, or her genetic makeup determine to what level her fetus may be affected by Zika infection? Other than avoiding the bite of a Zika-carrying mosquito and sexual transmission through a Zika-infected partner, are there other ways a pregnant woman can reduce her risk of having a baby affected by Zika? As with other advances in birth defects research on topics such as rubella, folic acid, Accutane, and valproic acid, the Teratology Society and its members have a critical role to play in providing answers to these questions and moving toward prevention of the adverse outcomes associated with Zika. In June 2017, scientists will meet again at the Teratology Society annual meeting to share the latest information on birth defects research and prevention. Again, we will have a special session on Zika virus—where we will continue to work together to better understand how the virus damages the fetus and ways to prevent the effects of Zika on the developing fetus.
While I pause to marvel at what a difference a year can make in this field, I am also re-energized. I wonder what a difference 2017 will make, especially if researchers from various disciplines work together. January’s Birth Defects Prevention Month is upon us. The time is now to come together, research, and #Prevent2Protect.
About the Author
Sonja A. Rasmussen, MD, MS, is editor-in-chief of the Morbidity and Mortality Weekly Report (MMWR) Series and director of the Centers for Disease Control and Prevention’s Division of Public Health Information Dissemination in the Center for Surveillance, Epidemiology, and Laboratory Services.
About the Teratology Society
Scientists interested or are already involved in research related to topics mentioned in this blog are encouraged to join the Teratology Society, the premier source for cutting-edge research and authoritative information related to birth defects and developmentally-mediated disorders. Teratology Society members include those specializing in cell and molecular biology, developmental biology and toxicology, reproduction and endocrinology, epidemiology, nutritional biochemistry, and genetics, as well as the clinical disciplines of prenatal medicine, pediatrics, obstetrics, neonatology, medical genetics, and teratogen risk counseling. In addition, the Teratology Society publishes the scientific journal, Birth Defects Research. Learn more at www.Teratology.org. Find the Teratology Society on LinkedIn, Facebook, and Twitter.
2016
Published December 2016
By Lauren Walker, BS, and Michael Collins, PhD
Annually, approximately 50 million people in the United States are affected by acne vulgaris. Acne can develop at any age, though it is most common in teenagers and young adults. Individuals suffering from moderate to severe acne such as nodular cystic acne often turn to prescription medications for relief. One class of treatments, retinoids, works to prevent the development of new blemishes while helping to clear existing ones. Vitamin A (retinol) and its derivatives are essential for proper embryo development. However, orally taken retinoid treatments have been associated with severe teratogenicity risks when taken during pregnancy, such as severe structural malformations of the newborn’s craniofacial, cardiac, thymic, and central nervous systems. As a result, they have traditionally been available by prescription-only with strict controls regarding prescribing to women who could get pregnant. All that changed in July 2016. Differin® Gel 0.1% is now the first retinoid-based acne medication to be approved by the FDA for over-the-counter (OTC) use. But before you start calling all of the pregnant women you know warning them about this gel, let’s take a closer look as to why this change occurred…
Acne 101
Having acne stinks—most people know this. But what is less known is that it can impart adverse psychological effects on acne patients such as poor self-image and anxiety. Acne blemishes form when hair follicles in the skin become congested. Generally, blemishes occur on the face, neck, back, chest, and/or shoulders and can range from mild pimples to painful cysts that form deep under the surface of the skin—as is the case with cystic acne. Acne blemishes can also cause permanent scarring. Prescription and OTC options with a variety of active ingredients are available to treat acne.
The Rise of Retinoids
One mainstay of prescription treatments is vitamin A (retinol) and vitamin A derivatives (i.e., retinoic acid, isotretinoin), often referred to as “retinoids.” In the early 1980s, isotretinoin—the active ingredient in Accutane—was identified as a highly effective treatment for severe nodular cystic acne. Isotretinoin is a natural metabolite of vitamin A commonly found in the blood stream. Its discovery as a cystic acne treatment was also accompanied by reports of adverse pregnancy outcomes including increased risk of spontaneous abortion and severe birth defects in newborns. The characteristic birth defect pattern associated with Accutane teratogenicity involved defects of the craniofacial, cardiac, thymic and central nervous system structures, as was first described in 1985 by Dr. Edward Lammer. Accutane and similar drugs were subsequently classified by the FDA as a Pregnancy Category X drug, indicating that female patients who are or may become pregnant or who are breast-feeding should not be prescribed or use the drugs. In light of this history why, then, has a retinoid drug been approved for OTC use?
Re-routing Retinoids
A chemical’s route of exposure is instrumental in dictating its toxicity. Just ask Teratology Society colleague, Dr. Sarah G. Obican, who was on the review board that approved Differin® for over-the-counter use. “The route has to do at least in part with the dose that reaches the fetus,” she explains.
In the case of retinoids, the bioavailability of the compounds and their associated teratogenic effects are mediated by how the mother is exposed. Oral treatments with retinoids lead to rapid absorption into the blood via the digestive tract. Once taken up by the blood, retinoids can be quickly distributed through the body and ultimately cross the blood-placental barrier and reach the developing embryo. In contrast, topical retinoid applications feature significantly reduced and slower systemic absorption through the skin into the bloodstream. Overall, much less active ingredient enters the blood and thus topical application offers a less risky acne treatment option. All tested retinoids have significantly less absorption through the dermal route than through the gastrointestinal route. But what if the patient’s skin barrier is compromised as is frequently the case for cystic acne sufferers? The structure of the active ingredient in Differin®, adapalene, includes a phenoxy-adamantyl group, which results in even lower percutaneous transport compared to other retinoids and consequently reduced blood concentrations. “There is a very large margin of safety between the concentrations achievable in patients, even with overuse of the drug, and concentrations associated with adverse developmental events,” assures Dr. Anthony Scialli, another Society colleague who participated in the approval process.
To Use or Not to Use During Pregnancy
Topically applied retinoids—first approved for prescription use by the FDA in 1971—have become one of the pillars of first-line acne treatment. Despite the reduced teratogenicity risk posed by Differin® Gel 0.1%, women who are pregnant, planning to become pregnant, or breast-feeding should still consult a doctor before use. “Together doctors and patients can make a decision to use by reviewing the needs of the woman, discussing treatment options plus understanding all of the available data,” says Dr. Obican.
Retinoids and Beyond
Since the identification of isotretinoin teratogenicity, studies of the relationship between different retinoid compounds and adverse pregnancy outcomes have provided a broader understanding of the biological impact of retinoids. Most critically, such research has identified less risky retinoid compounds and less risky treatment approaches. Considering that about half of all pregnancies in the United States are unplanned, it is by no means trivial that such alternative treatments are now available. Retinoids are only one of the many chemicals that are the subject of current research aimed at identifying and understanding how birth defects arise. As evidenced by the history of retinoid teratogenicity studies, birth defects research is vital in that it helps us to understand the risks posed by different chemicals and agents with the ultimate goal of preventing birth defects and protecting baby’s health.
About the Authors
Lauren Walker is a PhD Candidate studying environmental toxicology at the University of California, Riverside. Michael Collins is a Professor in the Interdepartmental Program in Molecular Toxicology and in Environmental Health Sciences at the University of California, Los Angeles.
About the Teratology Society
Scientists interested or are already involved in research related to topics mentioned in this blog are encouraged to join the Teratology Society, the premier source for cutting-edge research and authoritative information related to birth defects and developmentally-mediated disorders. Teratology Society members include those specializing in cell and molecular biology, developmental biology and toxicology, reproduction and endocrinology, epidemiology, nutritional biochemistry, and genetics, as well as the clinical disciplines of prenatal medicine, pediatrics, obstetrics, neonatology, medical genetics, and teratogen risk counseling. In addition, the Teratology Society publishes the scientific journal, Birth Defects Research. Learn more at www.Teratology.org. Find the Teratology Society on LinkedIn, Facebook, and Twitter.
Published April 2016

Teratology Society President
From its inception, the Teratology Society has contributed to some of the most important discoveries in birth defects research and has positively impacted the lives of countless children and families worldwide. As I look forward to our Society’s 56th Annual Meeting this June, I looked back at some of our field’s major accomplishments and was really impressed. Talk about return on investment in funding! Birth defects research has significantly advanced the science in fields such as developmental biology, genetics, and toxicology. Most importantly, it has improved the quality of life for the most vulnerable members of our communities. Today, we face new challenges, but I am confident that our field will again rise to the occasion and find solutions to current crises, such as the Zika virus, and future challenges in birth defects research, thereby impacting the lives of children and families for generations to come.
Averting an American Crisis
Founded by Drs. Josef Warkany, James G. Wilson and F. Clarke Fraser in 1960, the Teratology Society was intended to foster the exchange of information relating to birth defects including their nature, cause, mechanism and prevention. The timing couldn’t have been better. Within a year, the thalidomide crisis would hit Europe, and FDA reviewer and soon-to-be Society member, Dr. Francis Kelsey, would be hailed as a national hero for averting a similar crisis in the US by denying the approval of thalidomide.
At a time when the term, “translational research” was not even invented, the Society brought together a wide range of scientific and medical disciplines. While establishment of the Society formalized teratology research, Warkany, Wilson and Fraser had already contributed decades of research and hundreds of publications to the study of birth defects, in such areas as neural tube defects and other brain malformations, cleft lip and cleft palate, transplacental carcinogenesis; as well as defining the role of nutritional deficits, maternal diabetes, X-rays, DNA synthesis inhibitors, genes, and gene-environmental interactions in the development of birth defects. Fraser also established the link between medical genetics and experimental teratology and developed the field of genetic counselling.
In the wake of thalidomide, Warkany and Wilson had leadership roles in educating scientists and regulators in the principles of teratology, and best practices for study designs and fetal evaluation. They, along with Kelsey at the FDA, were key figures in establishing the first set of governmental guidelines and regulations to protect developing babies from radiation exposure (ICRP Recommendations) and to appropriately test pharmaceutical products (1966 FDA Guidelines).
Solidifying the Study of Teratology
Teratology Society members have always taken a true bedside-to-bench-to-bedside approach, covering the entire spectrum of birth defects research - from observing trends in human populations, to identifying mechanisms of action in the laboratory, to applying this knowledge to the prevention of birth defects from a wide range of causes. We continue that tradition today. Detailing all the contributions of all of our members would be impossible, but a review of some key highlights leaves no doubt about the importance of our work.
Accutane
Teratology Society members identified that the acne medication, Accutane, was a human teratogen, elucidated mechanisms of action, educated the public and physicians, and advocated for stricter warning labels and safeguards for use.
Fetal Alcohol Spectrum Disorder
Decades of research on fetal alcohol syndrome have been conducted by Society members, defining the critical periods of susceptibility, the various clinical manifestations of the condition, biomarkers of injury, and prevention strategies. Active research and educational programs are still being conducted today in this important area.
Folic Acid
The importance of proper nutrition for healthy development of the baby was investigated by Society co-founders, and continues to be vitally important today. Society members identified folic acid deficiency as a key factor in the development of neural tube (NT) defects, such as spina bifida, and have become staunch advocates for fortification of food with folic acid, an action that prevents 40–50% of NT defects each year in countries where fortification is required.
Maternal Diabetes
The identification of maternal diabetes as an important cause of birth defects and fetal harm was another important accomplishment of Society members, resulting in medical guidelines for proper control of diabetes during pregnancy.
Drug Labeling
Teratology Society members have collectively tested hundreds of chemicals and drugs for their potential to cause birth defects, identified mechanisms of action, and assessed human risk. The information goes directly into the drug label, helping physicians make important prescribing decisions for pregnant women with serious medical conditions. In addition, Society members have counseled thousands of pregnant women on their risks of birth defects based on genetic predisposition and inadvertent exposures, disciplines that sprang directly out of the efforts of our co-founder, Clarke Fraser, and other members.
Zika Virus, Microbiomes and the Future of Teratology
Currently, Teratology Society researchers are ferociously tackling the Zika virus epidemic, with active research to confirm the link with microcephaly, define susceptible periods during development, and develop prevention and mitigation strategies. We are also investigating how the maternal and fetal microbiome affect fetal development and postnatal health, and how in utero exposures can affect the health of the offspring throughout life and as adults through epigenetic mechanisms. Every day, we are identifying new genetic causes for birth defects and syndromes.
Looking to the future, I can see our work leading to advances such as gene therapy to prevent congenital defects, in utero corrective surgery, prevention of birth defects by solving the Zika puzzle, continuing to advocate for folic acid fortification, and so much more.
This important work deserves appropriate resources. More funding for teratology research is paramount in continuing to understand birth defects and devise prevention strategies, and so is the continued hard work and brain power of our Society members! Please consider joining us in June at our Annual Meeting and as a Society member.
About the Teratology Society
Scientists interested or are already involved in research related to topics mentioned in this blog are encouraged to join the Teratology Society, the premier source for cutting-edge research and authoritative information related to birth defects and developmentally-mediated disorders. The call for session proposals for the 2017 Annual Meeting deadline is April 15th. All scientists are encouraged to submit proposals.
Teratology Society members include those specializing in cell and molecular biology, developmental biology and toxicology, reproduction and endocrinology, epidemiology, nutritional biochemistry, and genetics, as well as the clinical disciplines of prenatal medicine, pediatrics, obstetrics, neonatology, medical genetics, and teratogen risk counseling. In addition, it publishes the scientific journal, Birth Defects Research. Learn more at www.Teratology.org.
Find the Teratology Society on Linkedin , Facebook, and Twitter.
Published March 2016

By Alan M. Hoberman, PhD, DABT, ATS, and Elise M. Lewis, PhD
Is the link between Zika and microcephaly real? Scientists will face an uphill battle proving cause and effect. Animal models can help.
Until a few months ago, microcephaly rarely came up in scientific discussions. Today, it’s front-page news. We’ve all seen or read the reports about microcephaly and its possible association with Zika, a virus spread by Aedes aegypti mosquitoes.
The numbers are concerning. Over 5,000 suspected cases of microcephaly have now been reported in Brazil after a major Zika outbreak surfaced in May, according to Brazilian public health authorities. Microcephaly cases have also been linked to an outbreak in French Polynesia that swept across the South Pacific island chain two years ago.
For those of us who research birth defects (in our case as reproductive and developmental toxicologists on the preclinical side) the information emerging from Brazil is confusing. For one, congenital anomalies don’t typically occur in clusters. Secondly, microcephaly has never been linked to a mosquito-borne virus until now, let alone one with a relatively benign profile.
By definition, microcephaly is a marked reduction in the size and development of the cranium and its contents. Microcephaly (Gr. mikros, small + kephale, head) is correlated with microencephaly (Gr. mikros, small + enkephalos, brain) due to the lack of pressure from the brain to expand the calvaria (i.e., skullcap) during fetal development. In humans, the most critical period of brain development is from Gestation Weeks 3 to 16, but abnormal development can still occur during the postnatal period. In the small animal models that we primarily work with, rodents and nonrodents, microcephaly does occur, but is difficult to diagnose. Numerous genetic factors and maternal exposure to hyperthermia, infectious agents such as cytomegalovirus, rubella and toxoplasma gondii, chemical agents, and high levels of irradiation during the fetal growth period have been linked to microcephaly, especially in animal models.
Its true incidence is also hard to deduce and reflective of how much debate there is on which head size actually constitutes microcephaly. Depending upon how one defines the condition, microcephaly can be as infrequent as 2 in every 10,000 births or as frequent as 12 in every 10,000 births. Microcephaly is defined by most clinicians and researchers as a head circumference (HC) of more than three standard deviations below the mean for gestational age and sex in defining microcephaly. The accuracy of the measurement can be influenced by the presence of fluid beneath the scalp and by the head shape.
Because we all have different head sizes at birth, a true microcephalic infant is difficult to diagnose. In certain instances, the head is just small without apparent developmental delays or life threatening conditions. But a baby with microcephaly may also suffer from seizures that shorten their life span, developmental delays, speech delays, hearing loss, and vision problems.
The Zika Puzzle
Even less is known about Zika, in part because its relatively benign nature has been of little interest to researchers until now. The Zika virus is related to the viruses that cause dengue, yellow fever, Japanese encephalitis, chikungunya, and West Nile. It was first recognized in the late 1940s. Until recently, it was thought to cause only mild symptoms in some cases and no symptoms in 80% of adult cases.
Given that the virus has been present in some parts of Africa for decades, one might wonder why we haven’t developed a vaccine sooner and how we let the virus, which is now evident in more than 30 countries, spiral out of control? The main reason is that Zika’s mostly mild symptoms never justified widespread vaccination. Vaccines are a great public health benefit, but they aren’t risk-free and so researchers and vaccine developers must always weigh the risk and benefits of contracting a given virus. In the case of smallpox or polio, developing a vaccine makes sense because the morbidity and mortality that occurs from contracting the virus vastly outweigh any adverse effects associated with the vaccine. Until now, Zika did not present such a public health problem.
The World Health Organization (WHO) and the US Centers for Disease Control and Prevention (CDC) have done the right thing in alerting the public about the potential health hazards of Zika infection, especially during pregnancy. In addition, the Teratology Society and MotherToBaby are taking a proactive approach to educating healthcare professionals and the general public by describing the epidemiology of the Zika virus as well as the malformations which have been proposed to be associated with Zika virus infection in pregnancy. Transmission of Zika virus seems to occur mostly via mosquitoes although the presence of the virus in semen or other body fluids may also lead to viral transmission. In either case, if it turns out that the real hazard is increased in pregnant women, then preventing infections during pregnancy may be important.
Efforts have also accelerated in recent days to develop a preventive vaccine, with the US National Institute of Allergy and Infectious Diseases’ Vaccine Research Center (VRC) leading the effort. The VRC has extensive experience working on vaccines for other mosquito-borne viruses such as West Nile virus, chikungunya virus, and dengue fever, and they developed one of the Ebola vaccine candidates that underwent clinical trials last year in Africa. Sanofi Pasteur, the vaccine division of French pharmaceutical giant Sanofi, which recently gained approval for the world’s first dengue vaccine, also just launched a project to develop a Zika vaccine.
This effort will take time, though. Nikos Vasilakis, an arbovirus researcher at University of Texas Medical Branch in Galveston and member of the Center for Biodefense and Emerging Infectious Diseases in Galveston told the BBC that it would take two years to develop a vaccine, but possibly 10 to 12 years to find one effective enough to be approved by regulators for public use.
Cause and effect
Given the high percentage of Zika infections that are probably going undetected, and the natural background incidence of microcephaly (the annual number of cases in Brazil prior to Zika), it is important now to move from association to understanding causation in order to prevent more cases of microcephaly and possibly other birth defects.
This is where basic research is needed in order to prove causation and lead us to the best ways to prevent birth defects following Zika exposure. To establish causation, researchers usually use the Bradford Hill criteria, otherwise known as Hill's criteria for causation. These are a group of minimal conditions necessary to provide adequate evidence of a causal relationship between incidence and possible consequence. They were established by the English epidemiologist Sir Austin Bradford Hill (1897–1991) in 1965.
The criteria are as follows:
-
Strength (effect size): A small association does not mean that there is not a causal effect, though the larger the association the more likely that it is causal.
-
Consistency (reproducibility) Consistent findings observed by different persons in different places with different samples strengthen the likelihood of an effect.
-
Specificity: Causation is likely if there is a very specific population at a specific site and disease with no other likely explanation. The more specific an association between a factor and an effect is, the bigger the probability of a causal relationship.
-
Temporality: The effect has to occur after the cause (and if there is an expected delay between the cause and expected effect, then the effect must occur after that delay).
-
Biological gradient: Greater exposure should generally lead to greater incidence of the effect. However, in some cases, the mere presence of the factor can trigger the effect. In other cases, an inverse proportion is observed: greater exposure leads to lower incidence.
-
Plausibility: A plausible mechanism between cause and effect is helpful (but Hill noted that knowledge of the mechanism is limited by current knowledge).
-
Coherence: Coherence between epidemiological and laboratory findings increases the likelihood of an effect. However, Hill noted that "... lack of such [laboratory] evidence cannot nullify the epidemiological effect on associations".
-
Experiment: "Occasionally it is possible to appeal to experimental evidence".[1]
-
Analogy: The effect of similar factors may be considered.
For the epidemiologist the gold standard would be a randomized control study of pregnant women exposed to Zika compared to a group of unexposed matched controls. Such a study could never be ethically conducted, however, because the fetus can never consent to the test. But a case-control study of women who have given birth to babies with microcephaly, compared with a group of women with babies without microcephaly could be examined for infection with Zika. In fact, such a study is already in the works and we should hopefully have results soon.
One can also glean clues from animal studies. For instance, pregnant animals could be infected with Zika virus and the outcomes monitored for signs of microcephaly. Of course, identifying an appropriate model would be necessary and basic research with the virus would be needed to determine if Zika is active in the exposed species. While prophylactic and therapeutic vaccines have different purposes (prevention vs. treatment), the study designs to test for potential reproductive or developmental effects are generally the same. The study designs can be combined or narrowed to focus on one or more aspects of the reproductive life cycle. Testing in conventional laboratory species may be appropriate, but the expectation is that the clinical product be tested in a sensitive species. In addition, the selected species should develop an immune response similar to that elicited by humans.
If causation is established, any treatment, whether it’s a preventive vaccine or an antiviral drug, must be found to be safe and effective. Since pregnant animals would be used for both aspects of this testing, understanding the background incidence of birth defects in the animal model and understanding the effects of exposure during pregnancy are of utmost importance.
Choosing the right animal models and doing the proper safety testing is key to accelerating the development of life-saving therapies. But the Zika response is very much a work in progress. Unlike the recent Ebola crisis in West Africa, where scientists had already gleaned a lot about the biology of the virus and had a vaccine candidate in the pipeline when the outbreak struck in 2014, scientists are starting from scratch on this one. They have yet to develop a rodent or large animal model for the disease, which will be needed to study the initial efficacy of any vaccine.
These are biting questions. The world wants answers. But the long-term solutions will take time and money and a collaborative response.
The latest Zika and microcephaly guidance from the CDC may be found here:
About the Authors
 Dr. Alan M. Hoberman is a 40-year veteran in toxicology who has specialized in reproductive and developmental toxicology for over 34 years. Currently, he is responsible for designing, supervising and evaluating reproductive, developmental, and juvenile toxicity studies throughout Charles River.Laboratories. He is a Diplomate of the American American Board of Toxicology, a fellow of the Academy of Toxicological Society and is the current Vice President-Elect of the Teratology Society. Dr. Hoberman has authored over 85 publications and presented 200 abstracts and lectures in the fields of reproductive and developmental toxicology, neurotoxicology, inhalation toxicology, photobiology and regulatory toxicology. This blog has also been posted on Dr. Hoberman's LinkedIn page.
Dr. Alan M. Hoberman is a 40-year veteran in toxicology who has specialized in reproductive and developmental toxicology for over 34 years. Currently, he is responsible for designing, supervising and evaluating reproductive, developmental, and juvenile toxicity studies throughout Charles River.Laboratories. He is a Diplomate of the American American Board of Toxicology, a fellow of the Academy of Toxicological Society and is the current Vice President-Elect of the Teratology Society. Dr. Hoberman has authored over 85 publications and presented 200 abstracts and lectures in the fields of reproductive and developmental toxicology, neurotoxicology, inhalation toxicology, photobiology and regulatory toxicology. This blog has also been posted on Dr. Hoberman's LinkedIn page.

Dr. Elise M. Lewis is a Study Director and Director, Reproductive and Neurobehavioral Toxicology at Charles River Laboratories’ safety and assessment site in Horsham, Pa. She has authored or co‑authored multiple publications in various areas of reproductive, developmental, and juvenile toxicology, and co-edited the first pediatric nonclinical drug testing book “Pediatric Non-Clinical Drug Testing: Principles, Requirements, and Practice.” Dr. Lewis also presents seminars and courses in animal research, toxicology and reproductive toxicology regularly for students from grade school through graduate school. Dr. Lewis is an active member of the Teratology Society, currently serving as Vice Chair of the Membership Committee.
About the Teratology Society
Scientists interested or are already involved in research related to topics mentioned in this blog are encouraged to join the Teratology Society, the premier source for cutting-edge research and authoritative information related to birth defects and developmentally-mediated disorders. Members include those specializing in cell and molecular biology, developmental biology and toxicology, reproduction and endocrinology, epidemiology, nutritional biochemistry, and genetics, as well as the clinical disciplines of prenatal medicine, pediatrics, obstetrics, neonatology, medical genetics, and teratogen risk counseling. In addition, it publishes the scientific journal, Birth Defects Research. Learn more at www.Teratology.org.
Find the Teratology Society on
About World Birth Defects Day
According to the International Clearninghouse for Birth Defects Surveillance and Research, an estimated 1 in 33 infants are affected by birth defects every year around the world, resulting in approximately 3.2 million birth defect-related disabilities. Along with more than 50 birth defects-related organizations around the world, the Teratology Society is helping to raise awareness of the important issue by participating in World Birth Defects Day. The commemorative day is observed yearly on March 3 and aims to highlight the importance of improving prevention strategies and research that will ultimately lead to a healthier society.

Published March 2016

By Sarah Običan, OBGYN
Teratology Society Council Member
Where should I even begin? As a physician, I’m concerned. As a scientist, I’m concerned. As a woman in her childbearing years, I’m concerned. While the vast majority of babies are born healthy and free of birth defects, the prevalence of certain birth defects are on the rise and the bottom line is–it’s concerning.
Just in the past few days, new evidence of increasing rates of gastroschisis has emerged. Gastroschisis is a serious birth defect where the intestines and sometimes other organs poke through an opening in the abdomen near the belly button. We don't understand why this birth defect occurs. There’s only one consistent risk factor identified in studies - moms who are younger are more likely to have a baby with this problem. But in recent years, the rate of this problem has been increasing and we don't know why.
How about antidepressants during pregnancy? That’s always a hot button issue that seems to gain a lot of press attention, from jumping to conclusions of its potential link to autism, to premature birth and everything in between. We still do not have concrete answers. What we do have is mounting evidence of the growing use of antidepressants among childbearing women. According to a study published just last week, about 15 percent of women aged 15–44 years filled at least one prescription for an antidepressant in a single year. Since much is not known about the safety of antidepressants in pregnancy, coupled with the known fact that about half of pregnancies are unplanned, research on the effects of antidepressants during pregnancy needs to be prioritized so women and their doctors have the information they need to make informed choices.
And then there’s Zika. It’s on the nightly news. It’s a discussion around the water cooler. It’s real and appears to not be going away anytime soon, especially as women all over the world prep to travel and cheer on their home countries in this Summer Olympic games in Brazil. The association between Zika virus during a woman’s pregnancy and microcephaly in her baby is rapidly evolving. Microcephaly is a life-threatening neurodevelopmental birth defect characterized by an affected infant’s smaller-than-normal head.
Science is Hope
As a Council member of the Teratology Society, a society of scientists dedicated to researching birth defects and developmentally-mediated disorders, and Board member of the Organization of Teratology Information Specialist’s (OTIS) MotherToBaby program, it goes without saying that I look forward to our annual conferences. One of the advantages of the conferences, held jointly, is that they bring together experts from many fields. Some are laboratory scientists who do genetic studies, others do animal research, some focus on epidemiology studies, while others see patients and talk to parents. All these smart minds at the same meeting -- both in the formal meeting rooms and in the conversations that happen in the hallway between sessions -- can come up with new ideas on how to tackle these important issues causing so much concern. These are groups that don't get the opportunity to exchange ideas on a regular basis. Submit your research abstract online (deadline: February 15th), take part in our annual meeting, and visit http://www.teratology.org/
I hope that by having the leaders in the studies on Zika virus from Brazil and the United States presenting to our group of researchers leads to an improved understanding of this association and improved care for mothers and their babies. I hope that by bringing the world’s leaders on antidepressant research together in one session will lead to new ways to study its use in pregnancy. I hope our scientists help answer that young mom left wondering what she could have done differently to avoid having her baby deal with the complications of gastroschisis. Bottom line—From hope comes motivation, which, as scientists, can lead to answers. Join the hope.
Scientists interested or are already involved in research related to topics mentioned in this blog are encouraged to join the Teratology Society, the premier source for cutting-edge research and authoritative information related to birth defects and developmentally-mediated disorders. Members include those specializing in cell and molecular biology, developmental biology and toxicology, reproduction and endocrinology, epidemiology, nutritional biochemistry, and genetics, as well as the clinical disciplines of prenatal medicine, pediatrics, obstetrics, neonatology, medical genetics, and teratogen risk counseling. In addition, it publishes the scientific journal, Birth Defects Research. Learn more at http://www.teratology.org/.
See Dr. Obican's blog on Linkedin and share it with your networks
Find the Teratology Society on LinkedIn, Facebook, and Twitter.
About The Author
Sarah G. Obican, MD, is an OBGYN, Maternal Fetal Medicine specialist at the University of South Florida. She’s currently a Councilor of the Teratology Society. She’s also on the Board of Directors of the Teratology Society’s sister organization, MotherToBaby, which provides evidence-based answers to questions about exposures during pregnancy and breastfeeding.
Published January 2016
By John Rogers, PhD
Teratology Society Past President
“County May Restrict Vaping,” “Vaping Burns Hole In Dad's LUNGS After E-Cigarette Spits Molten Nicotine Into His Throat,” and, perhaps one of the most disturbing, “Lab Tests Reveal Popular E-Cigarette Liquids Contain Harmful Chemicals.” These are just a few of the many headlines associated with vaping, otherwise known as electronic cigarette, or e-cigarette, use. Electronic cigarettes are also called electronic nicotine delivery systems (ENDS), the most descriptive term and the one I will use here. Why do I bring this up? Because, as a biologist and developmental toxicologist by profession, the risks associated with smoking and ENDS use is a professional interest. ENDS are here, they’re trending and they’re not going away anytime soon. So what does this mean for future generations? Particularly the ones growing in the wombs of many women exposed to the vaping culture? I’m calling on all scientists to take a closer look at the topic…first at the trends...
Where there’s smoke, there’s vaping.
Despite the disturbing headlines, vaping is on the rise. A 2013 study conducted by the Centers for Disease Control and Prevention (CDC) and Georgia State University, found that from 2010 to 2013, awareness of ENDS grew to 80% and use of them more than doubled among U.S. adults.[1] What may be equally, if not more disturbing is the early indication coming out of small studies that many pregnant women may escape to vape because of a presumption that ENDS are safer for the fetus than traditional smoking.[2] Their rising use raises a lot of questions, but with few answers yet.
What we do know is that ENDS are unregulated.
ENDS are currently unregulated and highly variable in what they deliver in their vapor, including levels of nicotine. Heavy metals such as tin, chromium and nickel have been found in aerosols from some ENDS, along with propylene glycol, glycerol, and flavorings. While e-cigarettes are being marketed as an aid to smoking cessation, none has been approved by the FDA for this use. The U.S. Preventive Services Task Force (USPSTF) [3] has concluded that nicotine replacement products (e.g., nicotine patch) and other pharmaceuticals for smoking cessation (varenicline and bupropion) have not been adequately tested for safety and efficacy during pregnancy, and the USPSTF and The American College of Obstetricians and Gynecologists [4] recommend that clinicians offer pregnancy-tailored smoking cessation counseling for smokers.
Research is lacking on using ENDS during pregnancy, but we know a lot about nicotine.
As nicotine delivery devices, ENDS deliver a known human developmental toxicant that targets, at least, the developing brain and lungs. The dose of nicotine delivered during vaping is not well known and likely highly variable. Considering dose and potency, nicotine is probably the most toxic of tobacco smoke constituents for the developing conceptus. Delivered doses of solvents, flavorings and contaminants are poorly understood. While ENDS aerosols contain far fewer chemicals than tobacco smoke, there are no comparative studies showing that they are safer for pregnancy.
How does the availability of ENDS affect decisions about smoking during pregnancy?
All other things being equal, if a pregnant woman cannot quit smoking during pregnancy, e-cigarettes might be a safer (but not safe) substitute. But all other things are not equal. Does having the ENDS option reduce the rate of smoking cessation, which is the desired outcome, or is it a step in the right direction? Further, most women smokers at least reduce the number of cigarettes per day during pregnancy, which may result in less nicotine exposure than switching to ENDS. But the most insidious effect of ENDS on pregnancy may originate years earlier; the sharp rise in use by previously non-smoking teens will result in increased nicotine addiction inevitably extending into pregnancy for many. So the number of exposed pregnancies may show a sharp rise in the future due to today’s successful marketing of ENDS to adolescents.
With so many questions and not many answers, clearly it’s time to kick this issue in the “butt,” examine it closely, and light the way for healthier pregnancies and society in general.
Scientists interested or are already involved in research related to topics mentioned in this blog are encouraged to join the Teratology Society, the premier source for cutting-edge research and authoritative information related to birth defects and developmentally-mediated disorders. Members include those specializing in cell and molecular biology, developmental biology and toxicology, reproduction and endocrinology, epidemiology, nutritional biochemistry, and genetics, as well as the clinical disciplines of prenatal medicine, pediatrics, obstetrics, neonatology, medical genetics, and teratogen risk counseling. In addition, it publishes the scientific journal, Birth Defects Research. Learn more at http://www.teratology.org/.
Find the Teratology Society on Linkedin: http://www.Linkedin.com/company/teratology-society, Facebook: http://www.Facebook.com/teratologysociety, and Twitter: https://twitter.com/teratologysoc
About the Author

John M. Rogers, PhD, has been a Teratology Society member since 1982 and is a past president. He is director of the Toxicity Assessment Division at the United States Environmental Protection Agency. His areas of expertise include developmental biology and toxicology, embryo/fetal physiology, epigenetics, maternal-fetal interactions, mechanisms of teratogenesis, maternal nutrition, and the developmental origins of health and disease.
[1] Brian A. King, Roshni Patel, Kimberly Nguyen, and Shanta R. Dube. “Trends in Awareness and Use of Electronic Cigarettes among U.S. Adults, 2010–2013.” Nicotine & Tobacco Research. First published online September 19, 2014, doi:10.1093/ntr/ntu191
[2] Mark, Katrina S. MD; et al. “Knowledge, Attitudes, and Practice of Electronic Cigarette Use Among Pregnant Women.” Journal of Addiction Medicine: July/August 2015 Volume 9 - Issue 4 - p 266–272
[3] Counseling and interventions to prevent tobacco use and tobacco-caused disease in adults and pregnant women: US Preventive Services Task Force reaffirmation recommendation statement Ann Intern Med 2009; 150:551–555
[4] American College of Obstetricians and Gynecologists. Smoking cessation during pregnancy. Committee Opinion No. 471, November 2010, Reaffirmed 2015.
This Blog is also available on Linkedin.
2015
Published June 2015

By Tacey E.K. White,
PhD
Teratology Society Vice President
In my day-to-day job I help pharmaceutical companies plan and interpret developmental toxicity information in animals, and assess the risks for these effects in women and men taking these medications. Understanding the mechanisms underlying effects in animals is fundamental to assessing whether effects would happen in humans. Part of my role also includes helping companies develop pregnancy labeling content for their medications. Sounds cut and dry, right? Not so much…
Major changes to the Food and Drug Administration’s (FDA) pregnancy labeling rule have just been finalized and will go into effect in June of this year. The changes will be challenging for companies to comply with, particularly as they revise labels that may be up to 14 years old. For decades the FDA had been aware of significant problems with the system used to categorize medications for use in pregnancy. As far back as 1992, the group I represent as Vice President, the Teratology Society, expressed concerns, noting that the Pregnancy Category system was often misinterpreted by prescribers, leading to poor patient counseling or under-treatment of serious diseases in pregnancy. The new FDA Pregnancy and Lactation Labeling Rule should be a major improvement with the inclusion of more descriptive risk statements, clinical and mode of action data in addition to animal data, and statements about the risks of untreated conditions. But, how do we bring this all together exactly?
Could the answers lie in Canada? Wait… That doesn’t make sense…or does it?!
It sure does! As the world’s premier group of scientists in the birth defects research field prepare to gather in Montreal, Canada at the end of this month for the Teratology Society’s 55th Annual Meeting, much of the excitement surrounds the pregnancy and lactation labeling workshop, “The Pregnancy and Lactation Labeling Rule: It's Here, Now What?” Workshop attendees will be able to hear directly from regulators on the changes to the labeling rule, tips for implementing these changes and how the content will be interpreted by clinicians-invaluable information as we all try to navigate these changes!
In addition, meeting symposia looking at mechanisms of birth defects and how they translate to humans will be crucially important, including “Are Human Skeletal Malformations the Result of Embryonic Arterial Dysgenesis?” This symposium brings together basic research in embryology with clinical observations on skeletal malformation etiology in humans, and is a prime example of the ability of the diverse membership of the Teratology Society to produce translational research on human birth defects. This information is directly applicable to my own basic research on developmental toxicity of the anti-malarial agent, artesunate, in particular the ability to make predictions about human risk for the skeletal defects observed in animal studies.
What? You want genetics, you say? We’ve got that covered too! The “Genetic and Environment Interactions of Common Malformations” symposium explores the contribution of genetics and various environmental factors to the development of several common malformations including congenital club foot, heart defects and infantile hydrocephalus. The information allows clinicians to develop and communicate prevention strategies to patients who may be at high risk for having babies with these malformations based on family history. The information is also important for basic researchers seeking to understand the mechanisms underlying the production of these birth defects.
My Big, Fat Scientific Family
The cross-disciplinary nature of our members (bench scientists, clinicians, epidemiologists, regulators) means that I can get to know researchers who are outside of my research sector, but who share a common goal of understanding and preventing birth defects and problems with fertility, all coming at it from different points of view. Without the Teratology Society, I would never have had the chance to meet these researchers, let alone discuss issues of mutual interest and potentially collaborate on projects that lead to important new discoveries. The society is small so, over the years, we have all established deep relationships with other members and getting together every year is like getting together with family. I hope you will join us this year for what is shaping up to be a great meeting!
Scientists interested in learning more about the Teratology Society’s research, attending the annual meeting, as well as becoming a member are encouraged to visit http://www.teratology.org/. Members include those specializing in cell and molecular biology, developmental biology and toxicology, reproduction and endocrinology, epidemiology, nutritional biochemistry, and genetics, as well as the clinical disciplines of prenatal medicine, pediatrics, obstetrics, neonatology, medical genetics, and teratogen risk counseling. In addition, it publishes the scientific journal, Birth Defects Research.
Find the Teratology Society on Linkedin: http://www.Linkedin.com/company/teratology-society and on Facebook: http://www.Facebook.com/teratologysociety
Download a PDF version of this Blog.
About the Author

Tacey E.K. White, PhD, is the Teratology Society Vice President and a managing scientist of toxicology and mechanistic biology at Exponent, an engineering and scientific consulting firm.
Published March 2015

By Mary Alice Smith, PhD, Teratology Society President & Godfrey P. Oakley Jr., MD, Teratology Society Past President
Folic acid. Two words mentioned in a commercial for Raisin Bran or during a visit to the OB shortly after a woman finds out she’s pregnant. Maybe you’ve even noticed them on a bottle in the vitamin aisle at the pharmacy. The point is, they’re familiar words and they should be. Why? Chances are if you’re a healthy adult reading this blog, you may have folic acid to partially thank for your good health.
Folic acid deficiency is known to be the primary cause of neural tube defects unless it has been prevented by folic acid fortification. Thus, fortifying foods supplies women of reproductive age enough folic acid to prevent two of the most serious birth defects: spina bifida and anencephaly. These two birth defects are major causes of neonatal, infant and childhood mortality, and disabilities around the world. It’s why we decided to highlight them during our participation in the first World Birth Defects Day this month. These birth defects are present and, for now, aren’t going away any time soon.
The Good News
Approximately 70 countries have folic acid fortification programs and prevent cases of spina bifida and anencephaly in an estimated 60,000 children a year. This is very good news.
When women of childbearing age take folic acid supplements before they get pregnant and during their pregnancies, the number of babies born with spina bifida or other neural tube defects decreases significantly—anywhere from 25–40%.
In 1996, the FDA required folic acid fortification of all “enriched” cereal grain products like flour and corn meal by Jan 1, 1998. In fact, the US was the first country to require folic acid fortification. It has been a public health triumph. Let’s look at how the research supports this sentiment:
-
It has saved about $10 billion in health care costs.
-
It has saved 1,300 babies each year in the US from developing spina bifida and anencephaly.
The Bad News
There are approximately 180,000 children around the world affected by preventable spina bifida and anencephaly because their governments have not required folic acid fortification. These are sad failures of public health policy that contribute to unnecessary neonatal, infant, and childhood mortality and morbidity. Think these public policy failures only happen outside of the US? Think again.
There is a hole in the dike in US fortification: corn masa flour. Despite $8 billion in sales with Hispanic Americans as the primary consumer of the product, it failed to be included in the 1996 FDA regulations. The lack of fortification of this key staple among the diets of so many Hispanic Americans is likely responsible for the continuing excess cases of spina bifida and anencephaly among this population.
In an article published earlier this year in the Centers for Disease Control and Prevention’s (CDC) Morbidity and Mortality Weekly Report (MMWR), there was a summary of CDC data showing that serum folate levels among Hispanic Americans is lower than other ethnic groups, just providing additional evidence of a problem. There is even an ongoing epidemic of spina bifida and anencephaly in Eastern Washington, where a large population of Hispanic Americans resides. The lack of folic acid fortification of corn masa flour is likely to be a major contributor to this epidemic.
The Thalidomide Epidemic Taught Us How to Plug the Dike
The excess cases of spina bifida and anencephaly among Hispanic births have the potential to be dramatically curbed. One way could be by working with the US Food and Drug Administration (FDA) to have folic acid determined a food additive. As it stands right now, there is no standard of identity for corn masa. According to current regulations, a study to show that folic acid placed in corn masa flour is stable must happen first. An alternative route could be to permit folic acid as an additive to corn masa flour. If the study shows that there is some loss of folic acid in the processing or storage, then a little more could be added to compensate.
You may recall the thalidomide epidemic of the 1950s and 1960s, during which 10,000 children were affected by the drug. Babies exposed prenatally to the medication were born with major malformations of the limbs, among other birth defects. The thalidomide epidemic led to major changes in drug regulations globally. Folic acid fortification has the potential to prevent neural tube defects in 18 times as many babies each year compared to the total number of babies deformed by thalidomide over the course of the entire epidemic. This makes one thing quite clear - the global impact of national folic acid fortification will have an even greater impact—by saving lives and preventing birth defects. It makes sense to encourage global health policy change when it comes to folic acid fortification.
On a side note, it is also worth pointing out that folic acid fortification eliminates folate deficiency anemia—another worldwide problem and yet another reason to fix this public health policy gap without delay. Having that said, preventing spina bifida and anencephaly should be reason enough to stop the stream of birth defects from continuing to flow right beneath us…
The Teratology Society adopted a resolution recommending that all countries fortify a commonly consumed food with folic acid to reduce the global burden of spina bifida. Scientists interested in learning more about the Teratology Society’s efforts as well as becoming a member are encouraged to visit http://www.teratology.org/. Members include those specializing in cell and molecular biology, developmental biology and toxicology, reproduction and endocrinology, nutritional biochemistry, genetics, and epidemiology, as well as the clinical disciplines of prenatal medicine, pediatrics, obstetrics, neonatology, medical genetics, and teratogen risk counseling. In addition, it publishes the scientific journal, Birth Defects Research, Parts A, B, and C.
View it on Facebook and Share this blog on LinkedIn or Facebook!
Download to download a PDF version of this Blog.
About The Authors

Mary Alice Smith, PhD, is the Teratology Society President and Professor of Developmental and Reproductive Toxicology at the University of Georgia.
Godfrey P. Oakley Jr., MD, is a Professor of Epidemiology at the Rollins School of Public Health at Emory University. He is often referred to as “the folic acid ambassador” for his work to highlight the effects of folic acid deficiency around the world.
Reference:
Williams, MSN, Cara T. Mai, DrPH, Joe Mulinare, MD, et al. Updated Estimates of Neural Tube Defects Prevented by Mandatory Folic Acid Fortification—United States, 1995–2011, MMWR Morb Mortal Wkly Rep 2015;64:1–5.
